Berberine ameliorates the LPS-induced imbalance of osteogenic and adipogenic differentiation in rat bone marrow-derived mesenchymal stem cells
- PMID: 33760123
- PMCID: PMC7974461
- DOI: 10.3892/mmr.2021.11989
Berberine ameliorates the LPS-induced imbalance of osteogenic and adipogenic differentiation in rat bone marrow-derived mesenchymal stem cells
Abstract
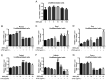
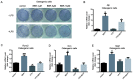
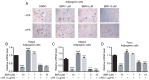
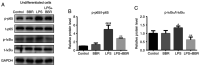
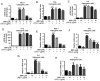
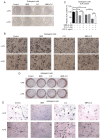
Lipopolysaccharide (LPS) from oral pathogenic bacteria is an important factor leading to alveolar bone absorption and the implant failure. The present study aimed to evaluate the modulation of berberine hydrochloride (BBR) on the LPS-mediated osteogenesis and adipogenesis imbalance in rat bone marrow-derived mesenchymal stem cells (BMSCs). Cell viability, osteoblastic and adipogenic differentiation levels were measured using the Cell Counting Kit-8 assay, alkaline phosphatase (ALP) staining and content assay, and oil red O staining, respectively. Reverse transcription-quantitative PCR and immunoblotting were used to detect the related gene and protein expression levels. In undifferentiated cells, BBR increased the mRNA expression levels of the osteoblastic genes (Alp, RUNX family transcription factor 2, osteocalcin and secreted phosphoprotein 1) but not the adipogenic genes (fatty acid binding protein 4, Adipsin and peroxisome proliferator-activated receptorγ). LPS-induced osteoblastic gene downregulation, adipogenic gene enhancement and NF-κB activation were reversed by BBR treatment. In osteoblastic differentiated cells, decreased ALP production by LPS treatment was recovered with BBR co-incubation. In adipogenic differentiated cells, LPS-mediated lipid accumulation was decreased by BBR administration. The mRNA expression levels of the pro-inflammatory factors (MCP-1, TNF-α, IL-6 and IL-1β) were increased by LPS under both adipogenic and osteoblastic conditions, which were effectively ameliorated by BBR. The actions of BBR were attenuated by compound C, suggesting that the role of BBR may be partly due to AMP-activated protein kinase activation. The results demonstrated notable pro-osteogenic and anti-adipogenic actions of BBR in a LPS-stimulated inflammatory environment. This indicated a potential role of BBR for bacterial infected-related peri-implantitis medication.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Berberine promotes bone marrow-derived mesenchymal stem cells osteogenic differentiation via canonical Wnt/β-catenin signaling pathway.Toxicol Lett. 2016 Jan 5;240(1):68-80. doi: 10.1016/j.toxlet.2015.10.007. Epub 2015 Oct 22. Toxicol Lett. 2016. PMID: 26478571
-
Sitagliptin regulates the AMPK/NF-κB signaling pathway to alleviate lipopolysaccharide-induced inflammatory responses and promote osteogenic differentiation in rat bone marrow mesenchymal stem cells.Arch Oral Biol. 2025 Jul;175:106253. doi: 10.1016/j.archoralbio.2025.106253. Epub 2025 Apr 6. Arch Oral Biol. 2025. PMID: 40215713
-
Tanshinone IIA promotes osteogenic differentiation potential and suppresses adipogenic differentiation potential of bone marrow mesenchymal stem cells.Mol Med Rep. 2024 Oct;30(4):177. doi: 10.3892/mmr.2024.13301. Epub 2024 Aug 12. Mol Med Rep. 2024. PMID: 39129299 Free PMC article.
-
Berberine as a promising natural compound for the treatment of periodontal disease: A focus on anti-inflammatory properties.J Cell Mol Med. 2021 Dec;25(24):11333-11337. doi: 10.1111/jcmm.17019. Epub 2021 Oct 30. J Cell Mol Med. 2021. PMID: 34719112 Free PMC article. Review.
-
Therapeutic Effects of Berberine on Liver Fibrosis are associated With Lipid Metabolism and Intestinal Flora.Front Pharmacol. 2022 Mar 2;13:814871. doi: 10.3389/fphar.2022.814871. eCollection 2022. Front Pharmacol. 2022. PMID: 35308208 Free PMC article. Review.
Cited by
-
Progress of bone marrow mesenchymal stem cell transplantation on neural plasticity in brain.Front Cell Dev Biol. 2025 Jun 10;13:1589169. doi: 10.3389/fcell.2025.1589169. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40556735 Free PMC article. Review.
-
An all-silk-derived bilayer hydrogel for osteochondral tissue engineering.Mater Today Bio. 2022 Nov 9;17:100485. doi: 10.1016/j.mtbio.2022.100485. eCollection 2022 Dec 15. Mater Today Bio. 2022. PMID: 36388458 Free PMC article.
-
Immunomodulatory effects of mesenchymal stem cell-conditioned media on lipopolysaccharide of Vibrio cholerae as a vaccine candidate.Stem Cell Res Ther. 2021 Nov 3;12(1):564. doi: 10.1186/s13287-021-02622-0. Stem Cell Res Ther. 2021. PMID: 34732259 Free PMC article.
-
Andrographis paniculata ameliorates estrogen deficiency-related osteoporosis by directing bone marrow mesenchymal stem cell fate.Ann Transl Med. 2023 Jan 31;11(2):52. doi: 10.21037/atm-22-1121. Ann Transl Med. 2023. PMID: 36819520 Free PMC article.
-
Natural compounds and mesenchymal stem cells: implications for inflammatory-impaired tissue regeneration.Stem Cell Res Ther. 2024 Feb 7;15(1):34. doi: 10.1186/s13287-024-03641-3. Stem Cell Res Ther. 2024. PMID: 38321524 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

