Expression of immunoglobulin G in human proximal tubular epithelial cells
- PMID: 33760139
- PMCID: PMC7974459
- DOI: 10.3892/mmr.2021.11966
Expression of immunoglobulin G in human proximal tubular epithelial cells
Abstract
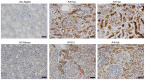
Proximal tubular epithelial cells (PTECs) have innate immune characteristics, and produce proinflammatory factors, chemokines and complement components that drive epithelial‑mesenchymal transition (EMT). Our previous studies revealed that human mesangial cells and podocytes were able to synthesize and secrete immunoglobulin (Ig)A and IgG, respectively. The aim of the present study was to evaluate the expression of Igs in PTECs. Firstly, IgG was detected in the cytoplasm, the cell membrane and the lumen of PTECs in the normal renal cortex by immunohistochemistry. Secondly, Igγ gene transcription and V(D)J recombination were detected in single PTECs by nested PCR and Sanger sequencing. Thirdly, Igγ, Igκ and Igλ were clearly detected in an immortalized PTEC line (HK‑2) by immunostaining and western blotting, in which RP215 (an antibody that predominantly binds to non‑B cell‑derived IgG) was used. In addition, Igγ, Igκ and Igλ gene transcripts, conservative V(D)J recombination in the Igγ variable region, recombination activating gene 1/2 and activation‑induced cytidine deaminase were all detected in HK‑2 cells. These data suggested that PTECs may express IgG in a similar manner to B cells. Furthermore, IgG expression was upregulated by TGF‑β1 and may be involved in EMT.
Keywords: proximal tubular epithelial cells; single cell; HK‑2; IgG; epithelial‑mesenchymal transition.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Single-cell RNA sequencing confirms IgG transcription and limited diversity of VHDJH rearrangements in proximal tubular epithelial cells.Sci Rep. 2020 Nov 12;10(1):19657. doi: 10.1038/s41598-020-75013-9. Sci Rep. 2020. PMID: 33184300 Free PMC article.
-
Expression of immunoglobulin G in human podocytes, and its role in cell viability and adhesion.Int J Mol Med. 2018 Jun;41(6):3296-3306. doi: 10.3892/ijmm.2018.3525. Epub 2018 Mar 1. Int J Mol Med. 2018. PMID: 29512722 Free PMC article.
-
Epithelial-mesenchymal transition and fibrosis are mutually exclusive reponses in shear-activated proximal tubular epithelial cells.FASEB J. 2012 Oct;26(10):4131-41. doi: 10.1096/fj.12-207324. Epub 2012 Jun 28. FASEB J. 2012. PMID: 22744866 Free PMC article.
-
Myeloma light chains induce epithelial-mesenchymal transition in human renal proximal tubule epithelial cells.Nephrol Dial Transplant. 2008 Mar;23(3):860-70. doi: 10.1093/ndt/gfm670. Epub 2007 Oct 12. Nephrol Dial Transplant. 2008. PMID: 17933841
-
HK2 Proximal Tubule Epithelial Cells Synthesize and Secrete Plasma Proteins Predominantly Through the Apical Surface.J Cell Biochem. 2017 Apr;118(4):924-933. doi: 10.1002/jcb.25786. Epub 2016 Dec 20. J Cell Biochem. 2017. PMID: 27862254
Cited by
-
The Expression of Non B Cell-Derived Immunoglobulins.Adv Exp Med Biol. 2024;1445:11-36. doi: 10.1007/978-981-97-0511-5_2. Adv Exp Med Biol. 2024. PMID: 38967747 Review.
-
Fluoxetine partially alleviates inflammation in the kidney of socially stressed male C57 BL/6 mice.FEBS Open Bio. 2023 Sep;13(9):1723-1736. doi: 10.1002/2211-5463.13670. Epub 2023 Aug 10. FEBS Open Bio. 2023. PMID: 37400956 Free PMC article.
-
Analysis of risk factors for tubular atrophy/interstitial fibrosis in primary membranous nephropathy.Medicine (Baltimore). 2025 Jul 25;104(30):e43455. doi: 10.1097/MD.0000000000043455. Medicine (Baltimore). 2025. PMID: 40725876 Free PMC article.
-
Lipotoxicity and immunometabolism in ischemic acute kidney injury: current perspectives and future directions.Front Pharmacol. 2024 Feb 23;15:1355674. doi: 10.3389/fphar.2024.1355674. eCollection 2024. Front Pharmacol. 2024. PMID: 38464721 Free PMC article. Review.
-
IgG expressed by renal tubular epithelial cells in epithelial mesenchymal transformation and interstitial fibrosis in diabetic kidney disease.Ren Fail. 2025 Dec;47(1):2458764. doi: 10.1080/0886022X.2025.2458764. Epub 2025 Feb 3. Ren Fail. 2025. PMID: 39901448 Free PMC article.
References
-
- Donadio ME, Loiacono E, Peruzzi L, Amore A, Camilla R, Chiale F, Vergano L, Boido A, Conrieri M, Bianciotto M, et al. Toll-like receptors, immunoproteasome and regulatory T cells in children with Henoch-Schonlein purpura and primary IgA nephropathy. Pediatr Nephrol. 2014;29:1545–1551. doi: 10.1007/s00467-014-2807-6. - DOI - PubMed
-
- Breda PC, Wiech T, Meyer-Schwesinger C, Grahammer F, Huber T, Panzer U, Tiegs G, Neumann K. Renal proximal tubular epithelial cells exert immunomodulatory function by driving inflammatory CD4+ T cell responses. Am J Physiol Renal Physiol. 2019;317:F77–F89. doi: 10.1152/ajprenal.00427.2018. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

