FXII regulates the formation of deep vein thrombosis via the PI3K/AKT signaling pathway in mice
- PMID: 33760144
- PMCID: PMC8018183
- DOI: 10.3892/ijmm.2021.4920
FXII regulates the formation of deep vein thrombosis via the PI3K/AKT signaling pathway in mice
Abstract
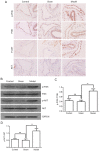
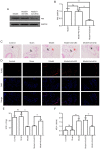
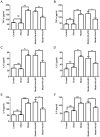
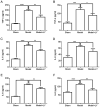
Deep vein thrombosis (DVT) is a common peripheral vascular disease, which may result in pulmonary embolism and is accompanied by endothelial injury. However, the pathogenesis of DVT remains unclear. Coagulation factor XII (FXII), as an important coagulation factor, has been reported to be closely associated with thrombosis. However, the association between FXII protein and DVT formation is not yet fully understood. The present study examined the effects of FXII protein on DVT formation and aimed to reveal the underlying mechanism. In the present study, histological characterization of the femoral vein tissue was examined by hematoxylin and eosin staining. The damage to the femoral vein tissue was examined by TUNEL assay. Superoxide dismutase (SOD) and malondialdehyde (MDA) concentrations were examined using ELISA. Tumor necrosis factor (TNF)‑α, interleukin (IL)‑6, IL‑8 and phosphoinositide 3‑kinase (PI3K)/AKT signaling were determined by ELISA, immunohistochemical staining and western blot analysis. The results demonstrated that thrombosis, FXII protein, cell apoptosis and the SOD concentrations were decreased, while the MDA concentrations were increased in mice with DVT compared with the control or sham groups. TNF‑α, IL‑6, IL‑8 and PI3K/AKT signaling was also upregulated in the mice with DVT. Furthermore, the knockdown of FXII significantly upregulated the SOD concentrations and downregulated thrombosis and cell apoptosis, as well as the MDA concentrations in mice with DVT. The knockdown of FXII also significantly downregulated the protein expression of TNF‑α, IL‑6 and IL‑8, and the activation of PI3K/AKT signaling. Additionally, LY294002 pre‑treatment markedly downregulated thrombosis and cell apoptosis and the MDA content, whereas it upregulated the SOD concentrations in mice with DVT. LY294002 pre‑treatment also significantly downregulated the TNF‑α, IL‑6 and IL‑8 protein levels. Taken together, the present study demonstrates that FXII protein promotes DVT via the activation of PI3K/AKT signaling by inducing an inflammatory response. Targeting FXII protein may thus prove to be a potential approach for the treatment of DVT.
Keywords: deep vein thrombosis; coagulation factor XII; PI3K/AKT signaling; inflammatory response.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
The combination of EGCG with warfarin reduces deep vein thrombosis in rabbits through modulating HIF-1α and VEGF via the PI3K/AKT and ERK1/2 signaling pathways.Chin J Nat Med. 2022 Sep;20(9):679-690. doi: 10.1016/S1875-5364(22)60172-9. Chin J Nat Med. 2022. PMID: 36162953
-
Epigallocatechin-3-gallate exerts protective effect on epithelial function via PI3K/AKT signaling in thrombosis.Microvasc Res. 2022 Nov;144:104408. doi: 10.1016/j.mvr.2022.104408. Epub 2022 Jul 22. Microvasc Res. 2022. PMID: 35878868
-
Downregulation of miR-125a-5p Promotes Endothelial Progenitor Cell Migration and Angiogenesis and Alleviates Deep Vein Thrombosis in Mice Via Upregulation of MCL-1.Mol Biotechnol. 2023 Oct;65(10):1664-1678. doi: 10.1007/s12033-023-00676-4. Epub 2023 Feb 4. Mol Biotechnol. 2023. PMID: 36738360
-
Factor XII: what does it contribute to our understanding of the physiology and pathophysiology of hemostasis & thrombosis.Thromb Res. 2010 Mar;125(3):210-5. doi: 10.1016/j.thromres.2009.11.028. Thromb Res. 2010. PMID: 20022081 Free PMC article. Review.
-
Advancements in research on the thrombo-inflammation mechanisms mediated by factor XII in ischemic stroke.J Thromb Thrombolysis. 2025 Jun;58(5):608-622. doi: 10.1007/s11239-025-03101-6. Epub 2025 Apr 26. J Thromb Thrombolysis. 2025. PMID: 40281266 Review.
Cited by
-
miR-2467-3p/ABLIM1 Axis Mediates the Formation and Progression of Deep Vein Thrombosis by Regulating Inflammation and Oxidative Stress.Int J Angiol. 2024 Feb 21;33(3):174-181. doi: 10.1055/s-0044-1779663. eCollection 2024 Sep. Int J Angiol. 2024. PMID: 39131807 Free PMC article.
-
Expression of miR-210-3p in the aqueous humor of patients with age-related cataracts and its effect on human lens epithelial cell injury induced by hydrogen peroxide.Arq Bras Oftalmol. 2023 Apr 17;87(5):e20220274. doi: 10.5935/0004-2749.2022-0274. eCollection 2023. Arq Bras Oftalmol. 2023. PMID: 39298734 Free PMC article.
-
Diagnostic and prognostic potential of long non-coding RNA NORAD in patients with acute deep vein thrombosis and its role in endothelial cell function.Thromb J. 2024 Jan 2;22(1):3. doi: 10.1186/s12959-023-00575-3. Thromb J. 2024. PMID: 38167080 Free PMC article.
-
Under the dual effect of inflammation and pulmonary fibrosis, CTD-ILD patients possess a greater susceptibility to VTE.Thromb J. 2024 Apr 4;22(1):34. doi: 10.1186/s12959-024-00599-3. Thromb J. 2024. PMID: 38576023 Free PMC article. Review.
-
Lysophosphatidic acid 2 alleviates deep vein thrombosis via protective endothelial barrier function.Open Med (Wars). 2025 Feb 6;20(1):20241137. doi: 10.1515/med-2024-1137. eCollection 2025. Open Med (Wars). 2025. PMID: 39927163 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

