miR‑320a‑3P alleviates the epithelial‑mesenchymal transition of A549 cells by activation of STAT3/SMAD3 signaling in a pulmonary fibrosis model
- PMID: 33760151
- PMCID: PMC7974326
- DOI: 10.3892/mmr.2021.11996
miR‑320a‑3P alleviates the epithelial‑mesenchymal transition of A549 cells by activation of STAT3/SMAD3 signaling in a pulmonary fibrosis model
Abstract
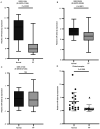
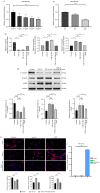
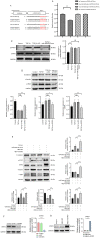
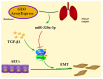
Pulmonary fibrosis (PF) is a common, chronic and incurable lung disease, in which the lungs become scarred over time. MicroRNAs (miRNAs/miRs) serve key roles in various biological processes, including cell proliferation, differentiation, apoptosis and the regulation of epithelial‑mesenchymal transition (EMT) process. The aim of the present study was to investigate the underlying mechanism of miR‑320a‑3p as a potential therapeutic target for PF. Clinical samples and microarray datasets collected from various databases were used to evaluate the expression of miR‑320a‑3p in PF. A549 cells were used to construct an EMT model of PF. A dual‑luciferase reporter assay system was used to identify target genes of miR‑320a‑3p. Western blot analysis and immunofluorescence staining were used to determine the roles of miR‑320a‑3p and its target genes in the EMT process in PF. The present study found that, compared with lung tissue of healthy control subjects, the expression of miR‑320a‑3p in lung tissue of PF patients was significantly reduced. The expression levels of miR‑320a‑3p decreased in TGF‑β1‑stimulated A549 cells in a time‑ and concentration‑dependent manner. The overexpression of miR‑320a‑3p suppressed EMT markers induced by TGF‑β1 in A549 cells and STAT3 was identified as a potential target gene of miR‑320a‑3p. Furthermore, the expression changes of miR‑320a‑3p and STAT3 were found to significantly affect the expression of phosphorylated SMAD3 in TGF‑β1‑stimulated A549 cells. Briefly, overexpression of miR‑320a‑3p could inhibit the EMT process in PF by downregulating STAT3 expression. The mechanism mediating these effects may partly involve crosstalk between the SMAD3 and STAT3.
Keywords: pulmonary fibrosis; epithelial‑mesenchymal transition; A549; miR‑320a‑3p; STAT3; SMAD3.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
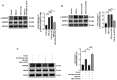
Similar articles
-
Therapeutic effects of conditioned medium from bone marrow-derived mesenchymal stem cells on epithelial-mesenchymal transition in A549 cells.Int J Mol Med. 2018 Feb;41(2):659-668. doi: 10.3892/ijmm.2017.3284. Epub 2017 Nov 24. Int J Mol Med. 2018. PMID: 29207055 Free PMC article.
-
miR‑221 targets HMGA2 to inhibit bleomycin‑induced pulmonary fibrosis by regulating TGF‑β1/Smad3-induced EMT.Int J Mol Med. 2016 Oct;38(4):1208-16. doi: 10.3892/ijmm.2016.2705. Epub 2016 Aug 11. Int J Mol Med. 2016. PMID: 27513632
-
Atrial natriuretic peptide: A novel mediator for TGF-β1-induced epithelial-mesenchymal transition in 16HBE-14o and A549 cells.Peptides. 2017 Apr;90:1-9. doi: 10.1016/j.peptides.2017.02.002. Epub 2017 Feb 13. Peptides. 2017. PMID: 28229930
-
Role of STAT3 in cancer cell epithelial‑mesenchymal transition (Review).Int J Oncol. 2024 May;64(5):48. doi: 10.3892/ijo.2024.5636. Epub 2024 Mar 15. Int J Oncol. 2024. PMID: 38488027 Free PMC article. Review.
-
New aspects of the epigenetic regulation of EMT related to pulmonary fibrosis.Eur J Pharmacol. 2023 Oct 5;956:175959. doi: 10.1016/j.ejphar.2023.175959. Epub 2023 Aug 2. Eur J Pharmacol. 2023. PMID: 37541361 Review.
Cited by
-
FOXM1 promotes the progression of non-small cell lung cancer by inhibiting miR-509-5p expression via binding to the miR-509-5p promoter region.Heliyon. 2024 Feb 27;10(5):e27147. doi: 10.1016/j.heliyon.2024.e27147. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38495135 Free PMC article.
-
Revisiting the role of MicroRNAs in the pathogenesis of idiopathic pulmonary fibrosis.Front Cell Dev Biol. 2024 Oct 16;12:1470875. doi: 10.3389/fcell.2024.1470875. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39479511 Free PMC article. Review.
-
The Inhibitory Effect of Phycocyanin Peptide on Pulmonary Fibrosis In Vitro.Mar Drugs. 2022 Nov 6;20(11):696. doi: 10.3390/md20110696. Mar Drugs. 2022. PMID: 36355019 Free PMC article.
-
miRNA/mRNA analysis of increased TGF-β pathways drive epithelial-mesenchymal transition and regulatory T cell differentiation.bioRxiv [Preprint]. 2025 Jun 26:2025.06.23.661210. doi: 10.1101/2025.06.23.661210. bioRxiv. 2025. PMID: 40667343 Free PMC article. Preprint.
-
miR-320-3p regulates apelin and TGF-β/SMAD3 signaling in hypobaric hypoxia exposed rats to induce skeletal muscle atrophy.J Physiol Biochem. 2025 Jun 11. doi: 10.1007/s13105-025-01100-y. Online ahead of print. J Physiol Biochem. 2025. PMID: 40498412
References
-
- Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. - DOI - PMC - PubMed
-
- Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: Potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1332. doi: 10.1016/S0002-9440(10)62351-6. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

