Piperazine ferulate attenuates high glucose‑induced mesangial cell injury via the regulation of p66Shc
- PMID: 33760157
- PMCID: PMC7985999
- DOI: 10.3892/mmr.2021.12013
Piperazine ferulate attenuates high glucose‑induced mesangial cell injury via the regulation of p66Shc
Abstract
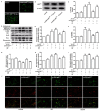
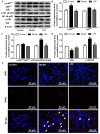
Diabetic nephropathy (DN) is a severe microvascular complication of diabetes. Hyperglycemia‑induced glomerular mesangial cells injury is associated with microvascular damage, which is an important step in the development of DN. Piperazine ferulate (PF) has been reported to exert protective effects against the progression of DN. However, whether PF prevents high glucose (HG)‑induced mesangial cell injury remains unknown. The aim of the present study was to investigate the effects of PF on HG‑induced mesangial cell injury and to elucidate the underlying mechanisms. Protein and mRNA expression levels were determined via western blot analysis and reverse transcription‑quantitative PCR, respectively. IL‑6 and TNF‑α levels were measured using ELISA. Reactive oxygen species levels and NF‑κB p65 nuclear translation were determined via immunofluorescence analysis. Apoptosis was assessed by measuring lactate dehydrogenase (LDH) release, as well as using MTT and flow cytometric assays. The mitochondrial membrane potential of mesangial cells was determined using the JC‑1 kit. The results revealed that LDH release were increased; however, cell viability and mitochondrial membrane potential were decreased in the HG group compared with the control group. These changes were inhibited after the mesangial cells were treated with PF. Moreover, PF significantly inhibited the HG‑induced production of inflammatory cytokines and the activation of NF‑κB in mesangial cells. PF also attenuated the HG‑induced upregulation of the expression levels of fibronectin and collagen 4A1. Furthermore, the overexpression of p66Src homology/collagen (Shc) abolished the protective effect of PF on HG‑induced mesangial cell injury. In vivo experiments revealed that PF inhibited the activation of inflammatory signaling pathways, glomerular cell apoptosis and mesangial matrix expansion in diabetic mice. Collectively, the present findings demonstrated that PF attenuated HG‑induced mesangial cells injury by inhibiting p66Shc.
Keywords: piperazine ferulate; inflammatory cytokine; diabetic nephropathy; apoptosis; p66 Src homology/collagen; mesangial cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Glucagon‑like peptide‑1 protects mouse podocytes against high glucose‑induced apoptosis, and suppresses reactive oxygen species production and proinflammatory cytokine secretion, through sirtuin 1 activation in vitro.Mol Med Rep. 2018 Aug;18(2):1789-1797. doi: 10.3892/mmr.2018.9085. Epub 2018 May 29. Mol Med Rep. 2018. PMID: 29845208
-
Fucoidan exerts protective effects against diabetic nephropathy related to spontaneous diabetes through the NF-κB signaling pathway in vivo and in vitro.Int J Mol Med. 2015 Apr;35(4):1067-73. doi: 10.3892/ijmm.2015.2095. Epub 2015 Feb 9. Int J Mol Med. 2015. PMID: 25672488
-
High glucose induces rat mesangial cells proliferation and MCP-1 expression via ROS-mediated activation of NF-κB pathway, which is inhibited by eleutheroside E.J Recept Signal Transduct Res. 2016;36(2):152-7. doi: 10.3109/10799893.2015.1061002. Epub 2015 Dec 7. J Recept Signal Transduct Res. 2016. PMID: 26644089
-
Protective effect of paeoniflorin in diabetic nephropathy: A preclinical systematic review revealing the mechanism of action.PLoS One. 2023 Sep 21;18(9):e0282275. doi: 10.1371/journal.pone.0282275. eCollection 2023. PLoS One. 2023. PMID: 37733659 Free PMC article.
-
The p66Shc Redox Protein and the Emerging Complications of Diabetes.Int J Mol Sci. 2023 Dec 20;25(1):108. doi: 10.3390/ijms25010108. Int J Mol Sci. 2023. PMID: 38203279 Free PMC article. Review.
Cited by
-
Targeting ferroptosis: a new therapeutic opportunity for kidney diseases.Front Immunol. 2024 Jul 3;15:1435139. doi: 10.3389/fimmu.2024.1435139. eCollection 2024. Front Immunol. 2024. PMID: 39021564 Free PMC article. Review.
-
The Role of p66Shc in Diabetes: A Comprehensive Review from Bench to Bedside.J Diabetes Res. 2022 Nov 24;2022:7703520. doi: 10.1155/2022/7703520. eCollection 2022. J Diabetes Res. 2022. PMID: 36465704 Free PMC article. Review.
-
Ferroptosis as a Novel Therapeutic Target for Diabetes and Its Complications.Front Endocrinol (Lausanne). 2022 Mar 29;13:853822. doi: 10.3389/fendo.2022.853822. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35422764 Free PMC article. Review.
-
Piperazine ferulate prevents high-glucose-induced filtration barrier injury of glomerular endothelial cells.Exp Ther Med. 2021 Oct;22(4):1175. doi: 10.3892/etm.2021.10607. Epub 2021 Aug 13. Exp Ther Med. 2021. PMID: 34504620 Free PMC article.
-
Ferroptosis: new insight into the mechanisms of diabetic nephropathy and retinopathy.Front Endocrinol (Lausanne). 2023 Aug 3;14:1215292. doi: 10.3389/fendo.2023.1215292. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37600716 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

