Long non‑coding RNA OIP5‑AS1 facilitates the progression of ovarian cancer via the miR‑128‑3p/CCNG1 axis
- PMID: 33760168
- PMCID: PMC8008222
- DOI: 10.3892/mmr.2021.12027
Long non‑coding RNA OIP5‑AS1 facilitates the progression of ovarian cancer via the miR‑128‑3p/CCNG1 axis
Abstract
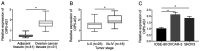
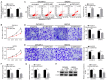
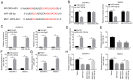
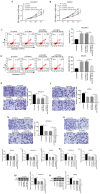
Long non‑coding RNA (LncRNA) o‑phthalaldehyde-interacting protein 5 antisense transcript 1 (OIP5‑AS1) serves major roles in the progression of various types of cancer. The present study investigated its biological function in ovarian cancer (OC) and its mechanisms. The levels of OIP5‑AS1, microRNA‑128‑3p (miR‑128‑3p) and cyclin G1 (CCNG1) were examined by reverse transcription‑quantitative PCR. Cell viability, apoptosis, migration and invasion were detected to analyze cellular progression. Glycolytic metabolism was assessed by detecting the levels of glucose consumption and lactate production. CCNG1 and hexokinase 2 protein levels were measured by western blotting. Dual‑luciferase reporter assay, RNA immunoprecipitation and RNA pull‑down assays were performed to affirm the interaction between two molecules. OIP5‑AS1 was found to be upregulated in OC tissues and cells. Knockdown of OIP5‑AS1 suppressed cell viability, migration, invasion and glycolysis while promoting apoptosis in OC cells. OIP5‑AS1 interacted with miR‑128‑3p and functioned as an oncogene by sequestering miR‑128‑3p. In addition, CCNG1 was a target gene for miR‑128‑3p and miR‑128‑3p regulated the CCNG1‑induced effects on OC cells by downregulating CCNG1. OIP5‑AS1 upregulated the expression of CCNG1 via targeting miR‑128‑3p. OIP5‑AS1 knockdown also inhibited tumor growth of OC in vivo by modulating the expression of miR‑128‑3p and CCNG1. Collectively, these data illustrated that the oncogenic role of OIP5‑AS1 in OC was associated with the miR‑128‑3p/CCNG1 axis at least in part. OIP5‑AS1 might be a probable diagnostic and therapeutic biomarker for the treatment of OC patients.
Keywords: long non‑coding RNA o‑phthalaldehyde‑interacting protein 5 antisense transcript 1; ovarian cancer; microRNA‑128‑3p; cyclin G1.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


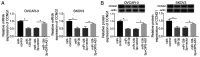

Similar articles
-
Noncoding RNAs in the Glycolysis of Ovarian Cancer.Front Pharmacol. 2022 Mar 30;13:855488. doi: 10.3389/fphar.2022.855488. eCollection 2022. Front Pharmacol. 2022. PMID: 35431949 Free PMC article. Review.
-
Potential role of lncRNA HULC/miR‑128‑3p/RAC1 axis in the inflammatory response during LPS‑induced sepsis in HMEC‑1 cells.Mol Med Rep. 2020 Dec;22(6):5095-5104. doi: 10.3892/mmr.2020.11601. Epub 2020 Oct 14. Mol Med Rep. 2020. PMID: 33174038 Free PMC article.
-
lncRNA SNHG22 sponges miR‑128‑3p to promote the progression of colorectal cancer by upregulating E2F3.Int J Oncol. 2021 Sep;59(3):71. doi: 10.3892/ijo.2021.5251. Epub 2021 Aug 9. Int J Oncol. 2021. PMID: 34368861 Free PMC article.
-
Long non‑coding RNA NEAT1 modifies cell proliferation, colony formation, apoptosis, migration and invasion via the miR‑4500/BZW1 axis in ovarian cancer.Mol Med Rep. 2020 Oct;22(4):3347-3357. doi: 10.3892/mmr.2020.11408. Epub 2020 Aug 4. Mol Med Rep. 2020. PMID: 32945505 Free PMC article.
-
Noncoding RNAs Interplay in Ovarian Cancer Therapy and Drug Resistance.Cancer Biother Radiopharm. 2022 Apr;37(3):186-198. doi: 10.1089/cbr.2021.0339. Epub 2022 Jan 4. Cancer Biother Radiopharm. 2022. PMID: 35133881 Review.
Cited by
-
miR‑135a‑5p inhibits tumor invasion by targeting ANGPT2 in gallbladder cancer.Mol Med Rep. 2021 Jul;24(1):528. doi: 10.3892/mmr.2021.12167. Epub 2021 May 26. Mol Med Rep. 2021. PMID: 34036386 Free PMC article.
-
Role of microRNAs in glycolysis in gynecological tumors (Review).Int J Oncol. 2023 May;62(5):63. doi: 10.3892/ijo.2023.5511. Epub 2023 Apr 13. Int J Oncol. 2023. PMID: 37052244 Free PMC article. Review.
-
Noncoding RNAs in the Glycolysis of Ovarian Cancer.Front Pharmacol. 2022 Mar 30;13:855488. doi: 10.3389/fphar.2022.855488. eCollection 2022. Front Pharmacol. 2022. PMID: 35431949 Free PMC article. Review.
-
Oxidative Stress and Inflammation in Cardiovascular Diseases and Cancer: Role of Non-coding RNAs.Yale J Biol Med. 2022 Mar 31;95(1):129-152. eCollection 2022 Mar. Yale J Biol Med. 2022. PMID: 35370493 Free PMC article. Review.
-
Identification of key lncRNAs associated with oxaliplatin resistance in colorectal cancer cells and isolated exosomes: From In-Silico prediction to In-Vitro validation.PLoS One. 2024 Oct 14;19(10):e0311680. doi: 10.1371/journal.pone.0311680. eCollection 2024. PLoS One. 2024. PMID: 39401197 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

