Tumor suppressor role of sFRP‑4 in hepatocellular carcinoma via the Wnt/β‑catenin signaling pathway
- PMID: 33760186
- PMCID: PMC7974405
- DOI: 10.3892/mmr.2021.11975
Tumor suppressor role of sFRP‑4 in hepatocellular carcinoma via the Wnt/β‑catenin signaling pathway
Abstract
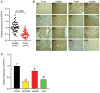
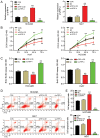
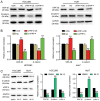
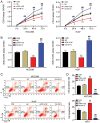
Hepatocellular carcinoma (HCC) is a malignant tumor located in the liver. Secreted frizzled‑related protein 4 (sFRP‑4) is associated with cancer occurrence, but the relationship between sFRP‑4 and HCC is not completely understood. The present study aimed to investigate the role and mechanism underlying sFRP‑4 in HCC. sFRP‑4 mRNA expression levels were determined via reverse transcription‑quantitative PCR and immunohistochemistry. The Cell Counting Kit‑8 assay was performed to evaluate HCCLM3 and Huh7 cell viability. Moreover, HCCLM3 and Huh7 cell proliferation were assessed using the BrdU ELISA assay kit, and cell apoptosis was measured via flow cytometry. Western blotting was conducted to measure β‑catenin and GSK‑3β protein expression levels. The results demonstrated that sFRP‑4 expression was significantly downregulated in HCC tissues and cells compared with adjacent healthy tissues and MIHA cells, respectively. Moreover, the results indicated that compared with the control group, sFRP‑4 overexpression inhibited HCC cell viability and proliferation, and accelerated HCC cell apoptosis. Furthermore, the results suggested that sFRP‑4 inhibited the Wnt/β‑catenin signaling pathway by upregulating GSK‑3β expression and downregulating β‑catenin expression, thus restraining the malignant behavior of HCC cells. In conclusion, the present study indicated that sFRP‑4 served a tumor suppressor role in HCC cells by restraining the Wnt/β‑catenin signaling pathway.
Keywords: secreted frizzled‑related protein 4; hepatocellular carcinoma; Wnt/β‑catenin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
SPAG5, the upstream protein of Wnt and the target of curcumin, inhibits hepatocellular carcinoma.Oncol Rep. 2023 Sep;50(3):172. doi: 10.3892/or.2023.8609. Epub 2023 Aug 4. Oncol Rep. 2023. PMID: 37539742 Free PMC article.
-
P7TP3 inhibits tumor development, migration, invasion and adhesion of liver cancer through the Wnt/β-catenin signaling pathway.Cancer Sci. 2020 Mar;111(3):994-1007. doi: 10.1111/cas.14243. Epub 2020 Feb 13. Cancer Sci. 2020. PMID: 31746531 Free PMC article.
-
M6A modification-mediated LIMA1 promotes the progression of hepatocellular carcinoma through the wnt-βcatenin/Hippo pathway.Cell Biol Toxicol. 2024 Dec 21;41(1):9. doi: 10.1007/s10565-024-09959-1. Cell Biol Toxicol. 2024. PMID: 39707043 Free PMC article.
-
Integrative analysis of aberrant Wnt signaling in hepatitis B virus-related hepatocellular carcinoma.World J Gastroenterol. 2015 May 28;21(20):6317-28. doi: 10.3748/wjg.v21.i20.6317. World J Gastroenterol. 2015. PMID: 26034368 Free PMC article. Review.
-
Improvement in the Current Therapies for Hepatocellular Carcinoma Using a Systems Medicine Approach.Adv Biosyst. 2020 Jun;4(6):e2000030. doi: 10.1002/adbi.202000030. Epub 2020 Apr 8. Adv Biosyst. 2020. PMID: 32529800 Review.
Cited by
-
20(S)- Protopanaxadiol suppresses hepatic stellate cell activation via WIF1 demethylation-mediated inactivation of the Wnt/β-catenin pathway.J Ginseng Res. 2023 Jul;47(4):515-523. doi: 10.1016/j.jgr.2022.05.005. Epub 2022 May 17. J Ginseng Res. 2023. PMID: 37397420 Free PMC article.
-
The role of novel adipokines in hepatocellular carcinoma progression: a mini review.Am J Cancer Res. 2024 Nov 15;14(11):5471-5485. doi: 10.62347/PZDM1736. eCollection 2024. Am J Cancer Res. 2024. PMID: 39659942 Free PMC article. Review.
-
SFRP4 Is a Potential Biomarker for the Prognosis and Immunotherapy for Gastric Cancer.J Oncol. 2022 Jul 5;2022:8829649. doi: 10.1155/2022/8829649. eCollection 2022. J Oncol. 2022. PMID: 35847366 Free PMC article.
-
Pancancer analysis of the interactions between CTNNB1 and infiltrating immune cell populations.Medicine (Baltimore). 2024 Nov 1;103(44):e40186. doi: 10.1097/MD.0000000000040186. Medicine (Baltimore). 2024. PMID: 39495984 Free PMC article.
-
SFRP4+IGFBP5hi NKT cells induced neural-like cell differentiation to contribute to adenomyosis pain.Front Immunol. 2022 Nov 30;13:945504. doi: 10.3389/fimmu.2022.945504. eCollection 2022. Front Immunol. 2022. PMID: 36532077 Free PMC article.
References
-
- Han J, Wang F, Lan Y, Wang J, Nie C, Liang Y, Song R, Zheng T, Pan S, Pei T, et al. KIFC1 regulated by miR-532-3p promotes epithelial-to-mesenchymal transition and metastasis of hepatocellular carcinoma via gankyrin/AKT signaling. Oncogene. 2019;38:406–420. doi: 10.1038/s41388-018-0440-8. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

