Personalized collection of plasma from healthy donors: A randomized controlled trial of a novel technology-enabled nomogram
- PMID: 33760230
- PMCID: PMC8251497
- DOI: 10.1111/trf.16389
Personalized collection of plasma from healthy donors: A randomized controlled trial of a novel technology-enabled nomogram
Abstract
Background: Source plasma is essential to support the growing demand for plasma-derived medicinal products. Supply is short, with donor availability further limited by the coronavirus disease 2019 (COVID-19) pandemic. This study examined whether a novel, personalized, technology-based nomogram was noninferior with regard to significant hypotensive adverse events (AEs) in healthy donors.
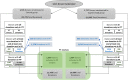
Study design and methods: IMPACT (IMproving PlasmA CollecTion) was a prospective, multicenter, double-blinded, randomized, controlled trial carried out between January 6 and March 26, 2020, in three U.S plasma collection centers. Donors were randomly assigned to the current simplified 1992 nomogram (control) or a novel percent plasma nomogram (PPN) with personalized target volume calculation (experimental). Primary endpoint was the rate of significant hypotensive AEs. Noninferiority (NI) was tested with a margin of 0.15%. Collected plasma volume was a secondary endpoint.
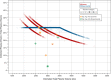
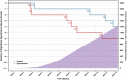
Results: A total of 3443 donors (mean [SD] BMI: 32 [7.74] kg/m2 ; 65% male) underwent 23,137 donations (median [range]: 6 [1-22] per subject). Ten significant hypotensive AEs were observed (six control; four experimental), with model-based AE incidence rate estimates (95% CI) of 0.051% (0.020%-0.114%) and 0.035% (0.010%-0.094%), respectively (p = .58). NI was met at an upper limit of 0.043% versus the predefined margin of 0.15%. There was no statistical difference between total AEs (all AE types: p = .32). Mean plasma volume collected was 777.8 ml (control) versus 841.7 ml (experimental); an increase of 63.9 ml per donation (8.2%; p < .0001).
Conclusion: This trial showed that a novel personalized nomogram approach in healthy donors allowed approximately 8% more plasma per donation to be collected without impairing donor safety.
Trial registration: ClinicalTrials.gov NCT04320823.
Keywords: donor safety; nomogram; plasma; plasmapheresis; source plasma.
© 2021 The Authors. Transfusion published by Wiley Periodicals LLC. on behalf of AABB.
Conflict of interest statement
Dr. Hartmann reports being employed by and holding equity interest in Haemonetics Corp. Mr. Ragusa reports being employed by and holding equity interest in Haemonetics Corp. and holding a related patent, assigned to Haemonetics Corp. (US 2018 / 0344910 A1). Dr. Burchardt, Dr. Manukyan, Dr. Popovsky, and Dr. Leitman report consulting fees from Haemonetics Corp. Dr. Leitman's opinions do not reflect the policy of the National Institutes of Health or the Department of Health and Human Services.
Figures
References
-
- Bult, J. The World Needs Plasma: From the USA and Other Countries. Plasma Protein Therapeutics Association. Presented at: Plasma Product Biotechnology Meeting; 2017. Available from: https://www.pptaglobal.org/images/presentations/2017/2017-_The_World_Nee.... Accessed August 16, 2020.
-
- FDA: Information About Immune Globulin (Human) Product Shortage . U.S. Food & Drug Administration; published August, 16, 2019. Available from: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologi.... Accessed July 27, 2020.
-
- FDA Issues Emergency Use Authorization for Convalescent Plasma as Potential Promising COVID–19 Treatment, Another Achievement in Administration's Fight Against Pandemic. U.S. Food and Drug Administration; published August 2020. Available from: https://www.fda.gov/news-events/press-announcements/fda-issues-emergency.... Accessed September 11, 2020.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

