Genetic analysis of carrier status in female members of Japanese hemophilia families
- PMID: 33760382
- PMCID: PMC8251972
- DOI: 10.1111/jth.15301
Genetic analysis of carrier status in female members of Japanese hemophilia families
Abstract
Background: Genetic characteristics and genetic carrier diagnosis in Japanese hemophilia female carriers have not been evaluated.
Objectives: To provide genetic information on Japanese hemophilia female carriers and demonstrate the advantages of genetic testing in carrier diagnosis.
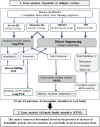
Methods: DNA sequencing combined with long polymerase chain reaction for inversion and multiplex ligation-dependent probe amplification for large mutations.
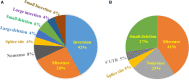
Results: Genetic analysis was performed in 69 male hemophiliac patients (48 hemophilia A [HA] and 21 hemophilia B [HB]) and 112 female family members (FFM) (80 from 50 families with HA and 32 from 22 families with HB). In 72 hemophiliac families, the identified F8 mutations were inversion (42%), missense (26%), and other variations (32%), while 74% of F9 mutations were point mutations. Among the 112 FFM, 53/80 (66%) with HA and 21/32 (66%) with HB were diagnosed genetically as carriers based on detection of heterozygous mutations. Low factor VIII activity (FVIII:C) levels (<50 IU/dL) were detected in only 10% of gene-confirmed carriers, suggesting that FVIII:C is not suitable for HA carrier prediction. Low FVIII/von Willebrand factor ratio (<0.9) was observed in 67% of gene-confirmed carriers. Half of the gene-confirmed HB carriers had low FIX:C (<60 IU/dL). Importantly, 32 mothers of 37 sporadic cases (86%) (24/27 [89%] HA and 8/10 [80%] HB) showed the relevant mutations, suggesting low incidence of de novo mutations in males.
Conclusions: This study is the first to provide genetic information on Japanese hemophilia female carriers. Gene analysis is the gold standard for carrier diagnosis as it well identifies undetected female carriers based on pedigree information and hemostatic measurements.
Keywords: carrier diagnosis; de novo mutation; female carriers; genetic analysis; hemophilia.
© 2021 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
K. Shinozawa was an endowed assistant professor funded by Baxter/Baxalta/Shire and CSL Behring (until March 2020). K. Amano has received honoraria from Chugai Pharmaceutical, Sanofi Genzyme, Bayer, Takeda Pharmaceutical, Novo Nordisk Pharma, CSL Behring, KM Biologics, and Pfizer. T. Hagiwara has received research funding from Sanofi Genzyme, and honoraria from Chugai Pharmaceutical, Bayer, Takeda Pharmaceutical, Novo Nordisk Pharma, CSL Behring, and Janssen Pharma. M. Bingo has received honoraria from Chugai Pharmaceutical, Sanofi Genzyme, Bayer, Takeda Pharmaceutical, Novo Nordisk Pharma, and CSL Behring. Y. Chikasawa has received honoraria from Chugai Pharmaceutical, Sanofi Genzyme, Bayer, Takeda Pharmaceutical, Novo Nordisk Pharma, and CSL Behring. H. Inaba declares no conflict of interest. E. Kinai has received honoraria and research grants from Chugai Pharmaceutical and also honoraria from Sanofi Genzyme, Bayer, Takeda Pharmaceutical, Novo Nordisk Pharma, and CSL Behring. K. Fukutake has received grants and personal fees from Takeda Co. Ltd. (Baxalta/Shire), Bayer, Pfizer, CSL Behring, Novo Nordisk, Sanofi S.A. (Biogen/Bioverativ), KM Biologics (Kaketsuken), and Chugai Pharmaceutical Co. Ltd, and grants from Japan Blood Products Organization, and CMIC Holdings Co., Ltd, and personal fees from SRL Inc., LSI Medience, Roche Diagnostics, Siemens, Sekisui Medical, Fujirebio Inc., Torii Pharmaceuticals, Octapharma, Sysmex, MSD outside the submitted work.
Figures
References
-
- McVey JH, Rallapalli PM, Kemball‐Cook G, et al. The European Association for Haemophilia and Allied Disorders (EAHAD) coagulation factor variant databases: important resources for haemostasis clinicians and researchers. Haemophilia. 2020;26:306‐313. - PubMed
-
- Graham JB, Rizza CR, Chediak J, et al. Carrier detection in hemophilia A: a cooperative international study. I. The carrier phenotype. Blood. 1986;67:1554‐1559. - PubMed
-
- Labarque V, Perinparajah V, Bouskill V, et al. Utility of factor VIII and factor VIII to von Willebrand factor ratio in identifying 277 unselected carriers of hemophilia A. Am J Hematol. 2017;92:E94‐E96. - PubMed
-
- Rizza CR, Rhymes IL, Austen DE, Kernoff PB, Aroni SA. Detection of carriers of haemophilia: a ‘blind’ study. Br J Haematol. 1975;30:447‐456. - PubMed
-
- Plug I, Mauser‐Bunschoten EP, Bröcker‐Vriends AHJT, et al. Bleeding in carriers of hemophilia. Blood. 2006;108:52‐56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

