Diagnostic accuracy of nasopharyngeal swab, nasal swab and saliva swab samples for the detection of SARS-CoV-2 using RT-PCR
- PMID: 33760699
- PMCID: PMC8006266
- DOI: 10.1080/23744235.2021.1903550
Diagnostic accuracy of nasopharyngeal swab, nasal swab and saliva swab samples for the detection of SARS-CoV-2 using RT-PCR
Abstract
Background: The current gold standard in coronavirus disease (COVID-19) diagnostics is the real-time reverse transcription-polymerase chain reaction (RT-PCR) assay for detecting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in nasopharyngeal swab (NPS) samples. Alternatively, nasal swab (NS) or saliva swab (SS) specimens are used, although available data on test accuracy are limited. We examined the diagnostic accuracy of NPS/NS/SS samples for this purpose.
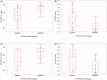
Methods: Ten patients were included after being tested positive for SARS-CoV-2 RT-PCR in NPS samples according to the National Institute of Infectious Disease guidelines. In comparison with this conventional diagnostic method, NPS/NS/SS samples were tested using the cobas 6800 systems RT-PCR device. To investigate the usefulness of the cobas method and the difference among sample types, the agreement and sensitivity were calculated. Five to six samples were collected over a total period of 5-6 d from each patient.
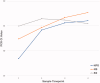
Results: Fifty-seven sets of NPS/NS/SS samples were collected, of which 40 tested positive for COVID-19 by the conventional method. Overall, the concordance rates using the conventional method were 86.0%/70.2%/54.4% for NPS/NS/SS samples (cobas); however, for samples collected up to and including on Day 9 after disease onset (22 negative and one positive specimens), the corresponding rates were 95.7%/87.0%/65.2%. The overall sensitivity estimates were 100.0%/67.5%/37.5% for NPS/NS/SS samples (cobas). For samples up to 9 d after onset, the corresponding values were 100.0%/86.4%/63.6%.
Conclusions: NS samples are more reliable than SS samples and can be an alternative to NPS samples. They can be a useful diagnostic method in the future.
Keywords: COVID-19 diagnostic test; nasal swab; nasopharyngeal swab; saliva.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- World Health Organization . Statement on the third meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of coronavirus disease (COVID-19) [Internet]; 2021. [cited 2021 Jan 14]. Available from: https://www.who.int/news-room/detail/01-05-2020-statement-on-the-third-m....
-
- World Health Organization . Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases. Geneva, Switzerland: World Health Organization; 2020. p. 1–7.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
