Neuronal death in pneumococcal meningitis is triggered by pneumolysin and RrgA interactions with β-actin
- PMID: 33760879
- PMCID: PMC7990213
- DOI: 10.1371/journal.ppat.1009432
Neuronal death in pneumococcal meningitis is triggered by pneumolysin and RrgA interactions with β-actin
Abstract
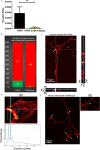
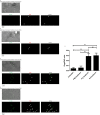
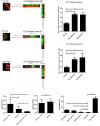
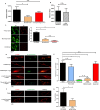
Neuronal damage is a major consequence of bacterial meningitis, but little is known about mechanisms of bacterial interaction with neurons leading to neuronal cell death. Streptococcus pneumoniae (pneumococcus) is a leading cause of bacterial meningitis and many survivors develop neurological sequelae after the acute infection has resolved, possibly due to neuronal damage. Here, we studied mechanisms for pneumococcal interactions with neurons. Using human primary neurons, pull-down experiments and mass spectrometry, we show that pneumococci interact with the cytoskeleton protein β-actin through the pilus-1 adhesin RrgA and the cytotoxin pneumolysin (Ply), thereby promoting adhesion and invasion of neurons, and neuronal death. Using our bacteremia-derived meningitis mouse model, we observe that RrgA- and Ply-expressing pneumococci co-localize with neuronal β-actin. Using purified proteins, we show that Ply, through its cholesterol-binding domain 4, interacts with the neuronal plasma membrane, thereby increasing the exposure on the outer surface of β-actin filaments, leading to more β-actin binding sites available for RrgA binding, and thus enhanced pneumococcal interactions with neurons. Pneumococcal infection promotes neuronal death possibly due to increased intracellular Ca2+ levels depending on presence of Ply, as well as on actin cytoskeleton disassembly. STED super-resolution microscopy showed disruption of β-actin filaments in neurons infected with pneumococci expressing RrgA and Ply. Finally, neuronal death caused by pneumococcal infection could be inhibited using antibodies against β-actin. The generated data potentially helps explaining mechanisms for why pneumococci frequently cause neurological sequelae.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- van de Beek D, de Gans J, Tunkel AR, Wijdicks EFM. Community-acquired bacterial meningitis in adults. N Eng J Med. 2006. January 5;354(1):44–53 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

