Plk4 triggers autonomous de novo centriole biogenesis and maturation
- PMID: 33760919
- PMCID: PMC7995200
- DOI: 10.1083/jcb.202008090
Plk4 triggers autonomous de novo centriole biogenesis and maturation
Abstract
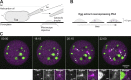
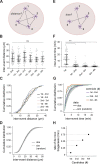
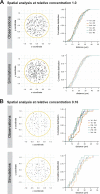
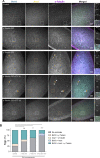
Centrioles form centrosomes and cilia. In most proliferating cells, centrioles assemble through canonical duplication, which is spatially, temporally, and numerically regulated by the cell cycle and the presence of mature centrioles. However, in certain cell types, centrioles assemble de novo, yet by poorly understood mechanisms. Herein, we established a controlled system to investigate de novo centriole biogenesis, using Drosophila melanogaster egg explants overexpressing Polo-like kinase 4 (Plk4), a trigger for centriole biogenesis. We show that at a high Plk4 concentration, centrioles form de novo, mature, and duplicate, independently of cell cycle progression and of the presence of other centrioles. Plk4 concentration determines the temporal onset of centriole assembly. Moreover, our results suggest that distinct biochemical kinetics regulate de novo and canonical biogenesis. Finally, we investigated which other factors modulate de novo centriole assembly and found that proteins of the pericentriolar material (PCM), and in particular γ-tubulin, promote biogenesis, likely by locally concentrating critical components.
© 2021 Nabais et al.
Figures








References
-
- Aydogan, M.G., Steinacker T.L., Mofatteh M., Gartenmann L., Wainman A., Saurya S., Conduit P.T., Zhou F.Y., Boemo M.A., and Raff J.W.. 2019. A free-running oscillator times and executes centriole biogenesis. BioRxiv. doi: (Preprint posted January 3, 2019)10.1101/510875v1 - DOI
-
- Bettencourt-Dias, M., Sinka R., Frenz L., and Glover D.M.. 2004. RNAi in Drosophila Cell Cultures. In Gene Silencing by RNA Interference: Technology and Application. Sohail M., editor. CRC Press, Inc.. pp. 147–166.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

