Top-down control of conditioned overconsumption is mediated by insular cortex Nos1 neurons
- PMID: 33761312
- PMCID: PMC8628615
- DOI: 10.1016/j.cmet.2021.03.001
Top-down control of conditioned overconsumption is mediated by insular cortex Nos1 neurons
Abstract
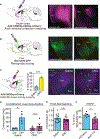
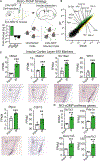
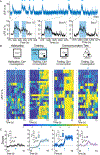
Associative learning allows animals to adapt their behavior in response to environmental cues. For example, sensory cues associated with food availability can trigger overconsumption even in sated animals. However, the neural mechanisms mediating cue-driven non-homeostatic feeding are poorly understood. To study this, we recently developed a behavioral task in which contextual cues increase feeding even in sated mice. Here, we show that an insular cortex to central amygdala circuit is necessary for conditioned overconsumption, but not for homeostatic feeding. This projection is marked by a population of glutamatergic nitric oxide synthase-1 (Nos1)-expressing neurons, which are specifically active during feeding bouts. Finally, we show that activation of insular cortex Nos1 neurons suppresses satiety signals in the central amygdala. The data, thus, indicate that the insular cortex provides top-down control of homeostatic circuits to promote overconsumption in response to learned cues.
Keywords: RNA sequencing; amygdala; associative learning; feeding; insular cortex; nitric oxide synthase-1.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

