FoxO1 suppresses Fgf21 during hepatic insulin resistance to impair peripheral glucose utilization and acute cold tolerance
- PMID: 33761350
- PMCID: PMC8529953
- DOI: 10.1016/j.celrep.2021.108893
FoxO1 suppresses Fgf21 during hepatic insulin resistance to impair peripheral glucose utilization and acute cold tolerance
Abstract
Fgf21 (fibroblast growth factor 21) is a regulatory hepatokine that, in pharmacologic form, powerfully promotes weight loss and glucose homeostasis. Although "Fgf21 resistance" is inferred from higher plasma Fgf21 levels in insulin-resistant mice and humans, diminished Fgf21 function is understood primarily via Fgf21 knockout mice. By contrast, we show that modestly reduced Fgf21-owing to cell-autonomous suppression by hepatic FoxO1-contributes to dysregulated metabolism in LDKO mice (Irs1L/L⋅Irs2L/L⋅CreAlb), a model of severe hepatic insulin resistance caused by deletion of hepatic Irs1 (insulin receptor substrate 1) and Irs2. Knockout of hepatic Foxo1 in LDKO mice or direct restoration of Fgf21 by adenoviral infection restored glucose utilization by BAT (brown adipose tissue) and skeletal muscle, normalized thermogenic gene expression in LDKO BAT, and corrected acute cold intolerance of LDKO mice. These studies highlight the Fgf21-dependent plasticity and importance of BAT function to metabolic health during hepatic insulin resistance.
Keywords: FGF21; FoxO1; brown adipose tissue; cold intolerance; glucose disposal; hepatic insulin resistance; hepatokine; insulin receptor substrate; peripheral insulin resistance.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.F.W. is an advisory board member of Housey Pharma (https://www.housey.com/).
Figures
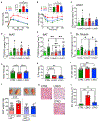
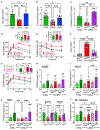



References
-
- Assini JM, Mulvihill EE, Burke AC, Sutherland BG, Telford DE, Chhoker SS, Sawyez CG, Drangova M, Adams AC, Kharitonenkov A, et al. (2015). Naringenin prevents obesity, hepatic steatosis, and glucose intolerance in male mice independent of fibroblast growth factor 21. Endocrinology 156, 2087–2102. - PubMed
-
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, and Maratos-Flier E (2007). Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 5, 426–437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

