Diverse and converging roles of ERK1/2 and ERK5 pathways on mesenchymal to epithelial transition in breast cancer
- PMID: 33761370
- PMCID: PMC8020482
- DOI: 10.1016/j.tranon.2021.101046
Diverse and converging roles of ERK1/2 and ERK5 pathways on mesenchymal to epithelial transition in breast cancer
Abstract
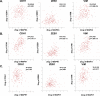
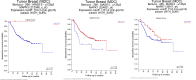
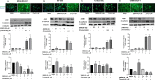
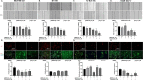
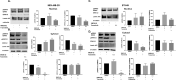
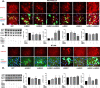
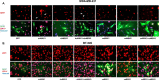
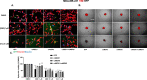
The epithelial to mesenchymal transition (EMT) is characterized by a loss of cell polarity, a decrease in the epithelial cell marker E-cadherin, and an increase in mesenchymal markers including the zinc-finger E-box binding homeobox (ZEB1). The EMT is also associated with an increase in cell migration and anchorage-independent growth. Induction of a reversal of the EMT, a mesenchymal to epithelial transition (MET), is an emerging strategy being explored to attenuate the metastatic potential of aggressive cancer types, such as triple-negative breast cancers (TNBCs) and tamoxifen-resistant (TAMR) ER-positive breast cancers, which have a mesenchymal phenotype. Patients with these aggressive cancers have poor prognoses, quick relapse, and resistance to most chemotherapeutic drugs. Overexpression of extracellular signal-regulated kinase (ERK) 1/2 and ERK5 is associated with poor patient survival in breast cancer. Moreover, TNBC and tamoxifen resistant cancers are unresponsive to most targeted clinical therapies and there is a dire need for alternative therapies. In the current study, we found that MAPK3, MAPK1, and MAPK7 gene expression correlated with EMT markers and poor overall survival in breast cancer patients using publicly available datasets. The effect of ERK1/2 and ERK5 pathway inhibition on MET was evaluated in MDA-MB-231, BT-549 TNBC cells, and tamoxifen-resistant MCF-7 breast cancer cells. Moreover, TU-BcX-4IC patient-derived primary TNBC cells were included to enhance the translational relevance of our study. We evaluated the effect of pharmacological inhibitors and lentivirus-induced activation or inhibition of the MEK1/2-ERK1/2 and MEK5-ERK5 pathways on cell morphology, E-cadherin, vimentin and ZEB1 expression. Additionally, the effects of pharmacological inhibition of trametinib and XMD8-92 on nuclear localization of ERK1/2 and ERK5, cell migration, proliferation, and spheroid formation were evaluated. Novel compounds that target the MEK1/2 and MEK5 pathways were used in combination with the AKT inhibitor ipatasertib to understand cell-specific responses to kinase inhibition. The results from this study will aid in the design of innovative therapeutic strategies that target cancer metastases.
Keywords: Breast cancer; ERK1/2; ERK5; Mesenchymal to epithelial transition (MET); Signaling.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors have no conflicts of interests.
Figures

References
-
- Chaffer C.L., Weinberg R.A. A perspective on cancer cell metastasis. Science (New York, N.Y.) 2011;331(6024):1559–1564. - PubMed
-
- Thiery J.P., Sleeman J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7(2):131–142. - PubMed
-
- Oka H., Shiozaki H., Kobayashi K., Inoue M., Tahara H., Kobayashi T., Takatsuka Y., Matsuyoshi N., Hirano S., Takeichi M. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 1993;53(7):1696–1701. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

