Super-rapid race for saving lives by developing COVID-19 vaccines
- PMID: 33761582
- PMCID: PMC8035961
- DOI: 10.1515/jib-2021-0002
Super-rapid race for saving lives by developing COVID-19 vaccines
Abstract
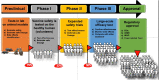
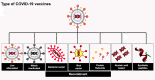
The pandemic of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected millions of people and claimed thousands of lives. Starting in China, it is arguably the most precipitous global health calamity of modern times. The entire world has rocked back to fight against the disease and the COVID-19 vaccine is the prime weapon. Even though the conventional vaccine development pipeline usually takes more than a decade, the escalating daily death rates due to COVID-19 infections have resulted in the development of fast-track strategies to bring in the vaccine under a year's time. Governments, companies, and universities have networked to pool resources and have come up with a number of vaccine candidates. Also, international consortia have emerged to address the distribution of successful candidates. Herein, we summarize these unprecedented developments in vaccine science and discuss the types of COVID-19 vaccines, their developmental strategies, and their roles as well as their limitations.
Keywords: COVID-19; SARS-CoV-2; vaccine development.
© 2021 Anusha Uttarilli et al., published by De Gruyter, Berlin/Boston.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
