Heme oxygenase-1 inducer hemin does not inhibit SARS-CoV-2 virus infection
- PMID: 33761605
- PMCID: PMC7881701
- DOI: 10.1016/j.biopha.2021.111384
Heme oxygenase-1 inducer hemin does not inhibit SARS-CoV-2 virus infection
Abstract
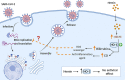
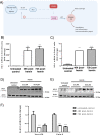
Antiviral agents with different mechanisms of action could induce synergistic effects against SARS-CoV-2 infection. Some reports suggest the therapeutic potential of the heme oxygenase-1 (HO-1) enzyme against virus infection. Given that hemin is a natural inducer of the HO-1 gene, the aim of this study was to develop an in vitro assay to analyze the antiviral potency of hemin against SARS-CoV-2 infection. A SARS-CoV-2 infectivity assay was conducted in Vero-E6 and Calu-3 epithelial cell lines. The antiviral effect of hemin, and chloroquine as a control, against SARS-CoV-2 virus infection was quantified by RT-qPCR using specific oligonucleotides for the N gene. Chloroquine induced a marked reduction of viral genome copies in kidney epithelial Vero-E6 cells but not in lung cancer Calu-3 cells. Hemin administration to the culture medium induced a high induction in the expression of the HO-1 gene that was stronger in Vero-E6 macaque-derived cells than in the human Calu-3 cell line. However, hemin treatment did not modify SARS-CoV-2 replication, as measured by viral genome quantification 48 h post-infection for Vero-E6 and 72 h post-infection for the Calu-3 lineages. In conclusion, although exposure to hemin induced strong HO-1 up-regulation, this effect was unable to inhibit or delay the progression of SARS-CoV-2 infection in two epithelial cell lines susceptible to infection.
Keywords: Antiviral effect; Heme oxygenase-1 induction; Hemin; SARS-CoV-2 infection.
Copyright © 2021 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Authors declare no conflicts of interest.
Figures

References
-
- Park J.-S., Choi H.-S., Yim S.-Y., Lee S.-M. Heme oxygenase-1 protects the liver from septic injury by modulating TLR4-mediated mitochondrial quality control in mice. Shock. 2018;50:209–218. 〈https://journals.lww.com/shockjournal/Fulltext/2018/08000/Heme_Oxygenas... - PubMed
-
- Huang H., Konduru K., Solovena V., Zhou Z.-H., Kumari N., Takeda K., Nekhai S., Bavari S., Kaplan G.G., Yamada K.M., Dhawan S. Therapeutic potential of the heme oxygenase-1 inducer hemin against Ebola virus infection. Curr. Trends Immunol. 2016;17:117–123. 〈https://pubmed.ncbi.nlm.nih.gov/28133423〉 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

