Correlation of autopsy pathological findings and imaging features from 9 fatal cases of COVID-19 pneumonia
- PMID: 33761714
- PMCID: PMC9282075
- DOI: 10.1097/MD.0000000000025232
Correlation of autopsy pathological findings and imaging features from 9 fatal cases of COVID-19 pneumonia
Abstract
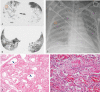
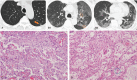
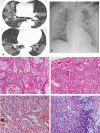
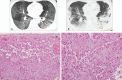
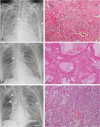
We aimed to investigate the relationship of radiological features and the corresponding pulmonary pathology of patients with Coronavirus Disease (COVID-19) pneumonia.In this multicenter study, serial chest CT and radiographic images from 9 patients (51-85 years old, 56% male) were reviewed and analyzed. Postmortem lungs were sampled and studied from these autopsies, with a special focus on several corresponding sites based on imaging features.The predominant pattern of pulmonary injury in these 9 cases was diffuse alveolar damage (DAD) and interstitial inflammation. Moreover, acute fibrinous exudates, organization, inflammatory cell infiltration, hyaline membranes, pulmonary edema, pneumocyte hyperplasia, and fibrosis were all observed. The histopathology features varied according to the site and severity of each lesion. In most of the 9 cases, opacities started from a subpleural area and peripheral structures were more severely damaged based on gross views and pathological examinations. Fibrosis could occur in early stages of infection and this was supported by radiological and pathological findings. The radiological features of COVID-19 pneumonia, at the critically ill stage, were diffuse ground-glass opacities with consolidation, interstitial thickening, and fibrous stripes, which was based in the fibrous tissue proliferation in the alveolar and interlobular septa, and filled alveoli with organizing exudation. Fungal and bacterial co-infections were also observed in 6 cases.Typical imaging features can be correlated with underlying pathological findings. Combining assessments of imaging features with pathological findings therefore can enhance our understanding of the histopathological mechanism of COVID-19 pneumonia, and facilitate early radiological diagnosis and prognosis estimation of COVID-19 pneumonia, which has important implications for the development of clinical targeted treatments and research related to COVID-19 pneumonia.
Copyright © 2021 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interests to disclose.
Figures
References
-
- International Committee on Taxonomy of Viruses. Naming the 2019 Coronavirus. Available at: https://talk.ictvonline.org/.[accessed February 11, 2020]
-
- World Health Organization. Weekly epidemiological update - December 29, 2020. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update---...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

