A retrospective study of alectinib versus ceritinib in patients with advanced non-small-cell lung cancer of anaplastic lymphoma kinase fusion in whom crizotinib treatment failed
- PMID: 33761908
- PMCID: PMC7988977
- DOI: 10.1186/s12885-021-08005-1
A retrospective study of alectinib versus ceritinib in patients with advanced non-small-cell lung cancer of anaplastic lymphoma kinase fusion in whom crizotinib treatment failed
Abstract
Background: Crizotinib is the approved treatment for advanced non-small cell lung cancers (NSCLCs) of anaplastic lymphoma kinase (ALK) fusion. Failure of crizotinib treatment frequently involves drug intolerance or resistance. Comparison of using second-generation ALK inhibitors in this setting remains lacking.
Methods: Sixty-five ALK-positive advanced NSCLC patients receiving second-generation ALK inhibitors following treatment failure of crizotinib were retrospectively analyzed for the therapeutic efficacy.
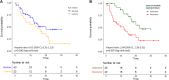
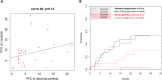
Results: Forty-three (66.2%) and 22 (33.8%) patients received alectinib and ceritinib, respectively. Comparing alectinib to ceritinib treatment: the 12-month progression-free survival (PFS) rate (61.0% [95% confidence interval, 47.1 to 78.9%] vs. 54.5% [95% CI, 37.3 to 79.9%]); the hazard ratio (HR) for disease progression or death, 0.61 (95% CI, 0.31-1.17; p = 0.135). Multivariate Cox regression showed ECOG PS (0-1 vs. 2-3 HR 0.09 [95% CI, 0.02-0.33]; p < 0.001) and cause of crizotinib treatment failure (resistance vs. intolerance HR 2.75 [95% CI, 1.26-5.99]; p = 0.011) were the independent predictors for the PFS of second-generation ALK inhibitors. Treatment of alectinib, compared to ceritinib, was associated with a lower incidence of CNS progression (cause-specific HR, 0.10; 95% CI 0.01-0.78; p = 0.029) and a higher efficacy in patients whose cause of crizotinib treatment failure was intolerance (HR 0.29 [95% CI, 0.08-1.06]; p = 0.050). The most commonly noted adverse events were elevated AST/ALT in 10 (23.3%) patients treated with alectinib and diarrhea in 8 (36.4%) patients treated with ceritinib.
Conclusion: Second-generation ALK inhibitors in crizotinib-treated patients showed a satifactory efficacy. Alectinib treatment demonstrated a CNS protection activity and a higher PFS in selected patients failing crizotinib treatment.
Keywords: ALK; Alectinib; CNS; Ceritinib; Crizotinib; NSCLC; Treatment failure.
Conflict of interest statement
None of the authors have any conflict of interest to disclose.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

