Delivery of cancer therapies by synthetic and bio-inspired nanovectors
- PMID: 33761944
- PMCID: PMC7987750
- DOI: 10.1186/s12943-021-01346-2
Delivery of cancer therapies by synthetic and bio-inspired nanovectors
Abstract
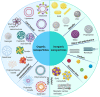
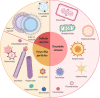
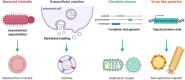
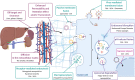
Background: As a complement to the clinical development of new anticancer molecules, innovations in therapeutic vectorization aim at solving issues related to tumor specificity and associated toxicities. Nanomedicine is a rapidly evolving field that offers various solutions to increase clinical efficacy and safety. MAIN: Here are presented the recent advances for different types of nanovectors of chemical and biological nature, to identify the best suited for translational research projects. These nanovectors include different types of chemically engineered nanoparticles that now come in many different flavors of 'smart' drug delivery systems. Alternatives with enhanced biocompatibility and a better adaptability to new types of therapeutic molecules are the cell-derived extracellular vesicles and micro-organism-derived oncolytic viruses, virus-like particles and bacterial minicells. In the first part of the review, we describe their main physical, chemical and biological properties and their potential for personalized modifications. The second part focuses on presenting the recent literature on the use of the different families of nanovectors to deliver anticancer molecules for chemotherapy, radiotherapy, nucleic acid-based therapy, modulation of the tumor microenvironment and immunotherapy.
Conclusion: This review will help the readers to better appreciate the complexity of available nanovectors and to identify the most fitting "type" for efficient and specific delivery of diverse anticancer therapies.
Keywords: Cancer therapy; Drug delivery; Nanomedicine; Nanoparticle; Targeting; Vectorization; Vesicle; Virus.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

