CACTUS: integrating clonal architecture with genomic clustering and transcriptome profiling of single tumor cells
- PMID: 33761980
- PMCID: PMC7988935
- DOI: 10.1186/s13073-021-00842-w
CACTUS: integrating clonal architecture with genomic clustering and transcriptome profiling of single tumor cells
Abstract
Background: Drawing genotype-to-phenotype maps in tumors is of paramount importance for understanding tumor heterogeneity. Assignment of single cells to their tumor clones of origin can be approached by matching the genotypes of the clones to the mutations found in RNA sequencing of the cells. The confidence of the cell-to-clone mapping can be increased by accounting for additional measurements. Follicular lymphoma, a malignancy of mature B cells that continuously acquire mutations in parallel in the exome and in B cell receptor loci, presents a unique opportunity to join exome-derived mutations with B cell receptor sequences as independent sources of evidence for clonal evolution.
Methods: Here, we propose CACTUS, a probabilistic model that leverages the information from an independent genomic clustering of cells and exploits the scarce single cell RNA sequencing data to map single cells to given imperfect genotypes of tumor clones.
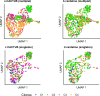
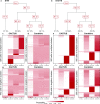
Results: We apply CACTUS to two follicular lymphoma patient samples, integrating three measurements: whole exome, single-cell RNA, and B cell receptor sequencing. CACTUS outperforms a predecessor model by confidently assigning cells and B cell receptor-based clusters to the tumor clones.
Conclusions: The integration of independent measurements increases model certainty and is the key to improving model performance in the challenging task of charting the genotype-to-phenotype maps in tumors. CACTUS opens the avenue to study the functional implications of tumor heterogeneity, and origins of resistance to targeted therapies. CACTUS is written in R and source code, along with all supporting files, are available on GitHub ( https://github.com/LUMC/CACTUS ).
Keywords: B cell receptor; Clonal evolution; Follicular lymphoma; Probabilistic graphical model; Single-cell sequencing; Somatic mutations.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

