Cross-kingdom inhibition of bacterial virulence and communication by probiotic yeast metabolites
- PMID: 33762022
- PMCID: PMC7992341
- DOI: 10.1186/s40168-021-01027-8
Cross-kingdom inhibition of bacterial virulence and communication by probiotic yeast metabolites
Abstract
Background: Probiotic milk-fermented microorganism mixtures (e.g., yogurt, kefir) are perceived as contributing to human health, and possibly capable of protecting against bacterial infections. Co-existence of probiotic microorganisms are likely maintained via complex biomolecular mechanisms, secreted metabolites mediating cell-cell communication, and other yet-unknown biochemical pathways. In particular, deciphering molecular mechanisms by which probiotic microorganisms inhibit proliferation of pathogenic bacteria would be highly important for understanding both the potential benefits of probiotic foods as well as maintenance of healthy gut microbiome.
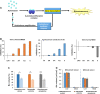
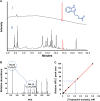
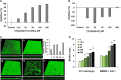
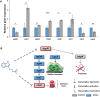
Results: The microbiome of a unique milk-fermented microorganism mixture was determined, revealing a predominance of the fungus Kluyveromyces marxianus. We further identified a new fungus-secreted metabolite-tryptophol acetate-which inhibits bacterial communication and virulence. We discovered that tryptophol acetate blocks quorum sensing (QS) of several Gram-negative bacteria, particularly Vibrio cholerae, a prominent gut pathogen. Notably, this is the first report of tryptophol acetate production by a yeast and role of the molecule as a signaling agent. Furthermore, mechanisms underscoring the anti-QS and anti-virulence activities of tryptophol acetate were elucidated, specifically down- or upregulation of distinct genes associated with V. cholerae QS and virulence pathways.
Conclusions: This study illuminates a yet-unrecognized mechanism for cross-kingdom inhibition of pathogenic bacteria cell-cell communication in a probiotic microorganism mixture. A newly identified fungus-secreted molecule-tryptophol acetate-was shown to disrupt quorum sensing pathways of the human gut pathogen V. cholerae. Cross-kingdom interference in quorum sensing may play important roles in enabling microorganism co-existence in multi-population environments, such as probiotic foods and the gut microbiome. This discovery may account for anti-virulence properties of the human microbiome and could aid elucidating health benefits of probiotic products against bacterially associated diseases. Video Abstract.
Keywords: Biofilms; Kluyveromyces marxianus; Microbiome; Probiotic microorganisms; Quorum sensing; Tryptophol acetate; Vibrio cholerae.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Tryptophol Acetate and Tyrosol Acetate, Small-Molecule Metabolites Identified in a Probiotic Mixture, Inhibit Hyperinflammation.J Innate Immun. 2023;15(1):531-547. doi: 10.1159/000529782. Epub 2023 Feb 21. J Innate Immun. 2023. PMID: 36809756 Free PMC article.
-
Parallel quorum-sensing system in Vibrio cholerae prevents signal interference inside the host.PLoS Pathog. 2020 Feb 14;16(2):e1008313. doi: 10.1371/journal.ppat.1008313. eCollection 2020 Feb. PLoS Pathog. 2020. PMID: 32059031 Free PMC article.
-
Potential role of probiotics in reducing Clostridioides difficile virulence: Interference with quorum sensing systems.Microb Pathog. 2021 Apr;153:104798. doi: 10.1016/j.micpath.2021.104798. Epub 2021 Feb 18. Microb Pathog. 2021. PMID: 33609647 Review.
-
Indole intercepts the communication between enteropathogenic E. coli and Vibrio cholerae.Gut Microbes. 2022 Jan-Dec;14(1):2138677. doi: 10.1080/19490976.2022.2138677. Gut Microbes. 2022. PMID: 36519445 Free PMC article.
-
Quorum Sensing, Biofilm, and Intestinal Mucosal Barrier: Involvement the Role of Probiotic.Front Cell Infect Microbiol. 2020 Sep 25;10:538077. doi: 10.3389/fcimb.2020.538077. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33102249 Free PMC article. Review.
Cited by
-
Probiotics Modulate the Ruminal Microbiome and Metabolite Availability to Enhance Rumen Barrier Function and Growth Performance in Goats Fed a High-Concentrate Diet.Probiotics Antimicrob Proteins. 2025 Jul 8. doi: 10.1007/s12602-025-10647-7. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 40627052
-
A metabolite from commensal Candida albicans enhances the bactericidal activity of macrophages and protects against sepsis.Cell Mol Immunol. 2023 Oct;20(10):1156-1170. doi: 10.1038/s41423-023-01070-5. Epub 2023 Aug 9. Cell Mol Immunol. 2023. PMID: 37553429 Free PMC article.
-
Bacterial-fungal metabolic interactions within the microbiota and their potential relevance in human health and disease: a short review.Gut Microbes. 2022 Jan-Dec;14(1):2105610. doi: 10.1080/19490976.2022.2105610. Gut Microbes. 2022. PMID: 35903007 Free PMC article. Review.
-
Tryptophol Acetate and Tyrosol Acetate, Small-Molecule Metabolites Identified in a Probiotic Mixture, Inhibit Hyperinflammation.J Innate Immun. 2023;15(1):531-547. doi: 10.1159/000529782. Epub 2023 Feb 21. J Innate Immun. 2023. PMID: 36809756 Free PMC article.
-
Quorum sensing in human gut and food microbiomes: Significance and potential for therapeutic targeting.Front Microbiol. 2022 Nov 25;13:1002185. doi: 10.3389/fmicb.2022.1002185. eCollection 2022. Front Microbiol. 2022. PMID: 36504831 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

