A partially randomised trial of pretomanid, moxifloxacin and pyrazinamide for pulmonary TB
- PMID: 33762075
- PMCID: PMC8009598
- DOI: 10.5588/ijtld.20.0513
A partially randomised trial of pretomanid, moxifloxacin and pyrazinamide for pulmonary TB
Abstract
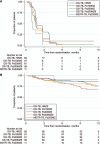
BACKGROUND: Treatment for TB is lengthy and toxic, and new regimens are needed.METHODS: Participants with pulmonary drug-susceptible TB (DS-TB) were randomised to receive: 200 mg pretomanid (Pa, PMD) daily, 400 mg moxifloxacin (M) and 1500 mg pyrazinamide (Z) for 6 months (6Pa200MZ) or 4 months (4Pa200MZ); 100 mg pretomanid daily for 4 months in the same combination (4Pa100MZ); or standard DS-TB treatment for 6 months. The primary outcome was treatment failure or relapse at 12 months post-randomisation. The non-inferiority margin for between-group differences was 12.0%. Recruitment was paused following three deaths and not resumed.RESULTS: Respectively 4/47 (8.5%), 11/57 (19.3%), 14/52 (26.9%) and 1/53 (1.9%) DS-TB outcomes were unfavourable in patients on 6Pa200MZ, 4Pa200MZ, 4Pa100MZ and controls. There was a 6.6% (95% CI -2.2% to 15.4%) difference per protocol and 9.9% (95%CI -4.1% to 23.9%) modified intention-to-treat difference in unfavourable responses between the control and 6Pa200MZ arms. Grade 3+ adverse events affected 68/203 (33.5%) receiving experimental regimens, and 19/68 (27.9%) on control. Ten of 203 (4.9%) participants on experimental arms and 2/68 (2.9%) controls died.CONCLUSION: PaMZ regimens did not achieve non-inferiority in this under-powered trial. An ongoing evaluation of PMD remains a priority.
CONTEXTE :: Le traitement de la TB est long et toxique et de nouveaux protocoles sont requis.
MÉTHODES :: Des patients atteints de TB pulmonaire sensible aux médicaments (DS-TB) ont été randomisés pour recevoir : 200 mg de prétomanide (Pa, PMD) quotidien, 400 mg de moxifloxacine (M) et 1500 mg de pyrazinamide (Z) pendant 6 mois (6Pa200MZ) ou pendant 4 mois (4Pa200MZ) ; 100 mg de PMD quotidien pendant 4 mois dans la même combinaison (4Pa100MZ) ; ou un traitement standard de DS-TB pendant 6 mois. Le résultat principal a été l’échec du traitement ou la rechute à 12 mois après la randomisation. La marge de non infériorité pour les différences entre groupes a été de 12,0%. Le recrutement a été mis en pause à la suite de trois décès et n’a pas été repris.
RÉSULTATS :: Quatre sur 47 (8,5%), 11 sur 57 (19,3%), 14 sur 52 (26,9%) et 1 sur 53 (1,9%) des résultats de DS-TB ont été défavorables pour 6Pa200MZ, 4Pa200MZ, 4Pa100MZ, et les témoins. Il y a eu une différence de 6,6% (IC 95% −2,2 à 15,4) par protocole et 9,9% (IC 95% −4,1 à 23,9) en réponse défavorable entre les témoins et 6Pa200MZ. Des effets secondaires de grade 3+ ont affecté 68 patients sur 203 (33,5%) recevant des protocoles expérimentaux et 19 sur 68 (27,9%) on control. Dix sur 203 (4,9%) participants en bras expérimental et 2 sur 68 (2,9%) on control sont décédés.
CONCLUSION :: Les protocoles Pa200MZ n’ont pas atteint la non infériorité dans cet essai sous-puissant. La priorité reste l’évaluation du PMD.
Figures

References
-
- World Health Organization. Geneva, Switzerland: WHO; 2019. Global tuberculosis report, 2019. WHO/CDS/TB/2019.15.
-
- Furin J, Cox H, Pai M. Tuberculosis. Lancet. 2019;393:1642–1656. - PubMed
-
- Stover CK, et al. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature. 2000;405:962–966. - PubMed
-
- Hu Y, Coates ARM, Mitchison DA. Comparison of the sterilising activities of the nitroimidazopyran PA-824 and moxifloxacin against persisting Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2008;12:69–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

