Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort
- PMID: 33762264
- PMCID: PMC8117443
- DOI: 10.1136/annrheumdis-2021-220272
Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort
Abstract
Introduction: In light of the SARS-CoV-2 pandemic, protecting vulnerable groups has become a high priority. Persons at risk of severe disease, for example, those receiving immunosuppressive therapies for chronic inflammatory cdiseases (CIDs), are prioritised for vaccination. However, data concerning generation of protective antibody titres in immunosuppressed patients are scarce. Additionally, mRNA vaccines represent a new vaccine technology leading to increased insecurity especially in patients with CID.
Objective: Here we present for the first time, data on the efficacy and safety of anti-SARS-CoV-2 mRNA vaccines in a cohort of immunosuppressed patients as compared with healthy controls.
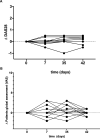
Methods: 42 healthy controls and 26 patients with CID were included in this study (mean age 37.5 vs 50.5 years). Immunisations were performed according to national guidelines with mRNA vaccines. Antibody titres were assessed by ELISA before initial vaccination and 7 days after secondary vaccination. Disease activity and side effects were assessed prior to and 7 days after both vaccinations.
Results: Anti-SARS-CoV-2 antibodies as well as neutralising activity could be detected in all study participants. IgG titres were significantly lower in patients as compared with controls (2053 binding antibody units (BAU)/mL ±1218 vs 2685±1102). Side effects were comparable in both groups. No severe adverse effects were observed, and no patients experienced a disease flare.
Conclusion: We show that SARS-CoV-2 mRNA vaccines lead to development of antibodies in immunosuppressed patients without considerable side effects or induction of disease flares. Despite the small size of this cohort, we were able to demonstrate the efficiency and safety of mRNA vaccines in our cohort.
Keywords: COVID-19; arthritis; rheumatoid; tumor necrosis factor inhibitors; vaccination.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Both BFH and SS received funding from Pfizer and other companies.
Figures

Comment in
-
Correspondence on 'Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort'.Ann Rheum Dis. 2021 Oct;80(10):e159. doi: 10.1136/annrheumdis-2021-220539. Epub 2021 May 24. Ann Rheum Dis. 2021. PMID: 34031031 No abstract available.
-
Correspondence on 'Immunogenicity and safety of anti-SARS-Cov-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort'.Ann Rheum Dis. 2021 Oct;80(10):e158. doi: 10.1136/annrheumdis-2021-220496. Epub 2021 May 28. Ann Rheum Dis. 2021. PMID: 34049854 No abstract available.
-
Correspondence on 'Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort'.Ann Rheum Dis. 2021 Oct;80(10):e160. doi: 10.1136/annrheumdis-2021-220736. Epub 2021 Jun 10. Ann Rheum Dis. 2021. PMID: 34112654 Free PMC article. No abstract available.
-
Correspondence on 'Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort'.Ann Rheum Dis. 2021 Oct;80(10):e161. doi: 10.1136/annrheumdis-2021-220898. Epub 2021 Jun 29. Ann Rheum Dis. 2021. PMID: 34187777 No abstract available.
-
Covid vaccination in patients with autoimmune diseases treated with mycophenolate: Let's think back to the recommendations.Autoimmun Rev. 2021 Oct;20(10):102908. doi: 10.1016/j.autrev.2021.102908. Epub 2021 Jul 16. Autoimmun Rev. 2021. PMID: 34274547 Free PMC article. No abstract available.
References
-
- Leipe J, Hoyer BF, Iking-Konert C, et al. [SARS-CoV-2 & rheumatic disease : Consequences of the SARS-CoV-2 pandemic for patients with inflammatory rheumatic diseases. A comparison of the recommendations for action of rheumatological societies and risk assessment of different antirheumatic treatments]. Z Rheumatol 2020;79:686–91. 10.1007/s00393-020-00878-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

