Bovine Immune Response to Vaccination and Infection with Leptospira borgpetersenii Serovar Hardjo
- PMID: 33762318
- PMCID: PMC8546708
- DOI: 10.1128/mSphere.00988-20
Bovine Immune Response to Vaccination and Infection with Leptospira borgpetersenii Serovar Hardjo
Abstract
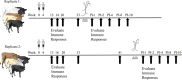
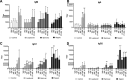
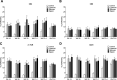
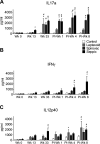
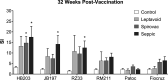
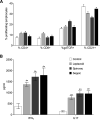
This study examined the humoral and cellular response of cattle vaccinated with two commercial leptospiral vaccines, Leptavoid and Spirovac, and a novel bacterin vaccine using Seppic Montanide oil emulsion adjuvant. Vaccination was followed by experimental challenge. All vaccinated cattle were protected from colonization of the kidney and shedding of Leptospira in urine, as detected by culture and immunofluorescence assay. Agglutinating antibody titers were detected in vaccinated cattle at 4 weeks following vaccination, with small anamnestic response detected following experimental challenge. Only animals vaccinated with the oil emulsion-adjuvanted bacterin produced significant IgG2 titers following vaccination, and nonvaccinated animals produced serum IgA titers after experimental challenge. CD4+ and γδ T cells from vaccinated cattle proliferated when cultured with antigen ex vivo Cellular responses included a marked proliferation of γδ T cells immediately following experimental challenge in vaccinated cattle and release of gamma interferon (IFN-γ), interleukin 17a (IL-17a), and IL-12p40 from stimulated cells. Proliferative and cytokine responses were found not just in peripheral mononuclear cells but also in lymphocytes isolated from renal lymph nodes at 10 weeks following experimental challenge. Overall, effects of leptospirosis vaccination and infection were subtle, resulting in only modest activation of CD4+ and γδ T cells. The use of Seppic Montanide oil emulsion adjuvants may shorten the initiation of response to vaccination, which could be useful during outbreaks or in areas where leptospirosis is endemic.IMPORTANCE Leptospirosis is an underdiagnosed, underreported zoonotic disease of which domestic livestock can be carriers. As a reservoir host for Leptospira borgpetersenii serovar Hardjo, cattle may present with reproductive issues, including abortion, birth of weak or infected calves, or failure to breed. Despite years of study and the availability of commercial vaccines, detailed analysis of the bovine immune response to vaccination and Leptospira challenge is lacking. This study evaluated immunologic responses to two efficacious commercial vaccines and a novel bacterin vaccine using an adjuvant chosen for enhanced cellular immune responses. Antigen-specific responsive CD4 and γδ T cells were detected following vaccination and were associated with release of inflammatory cytokines IFN-γ and IL-17a after stimulation. CD4 and γδ cells increased in the first week after infection and, combined with serum antibody, may play a role in clearance of bacteria from the blood and resident tissues. Additionally, these antigen-reactive T cells were found in the regional lymph nodes following infection, indicating that memory responses may not be circulating but are still present in regional lymph nodes. The information gained in this study expands knowledge of bovine immune response to leptospirosis vaccines and infection. The use of oil emulsion adjuvants may enhance early immune responses to leptospiral bacterins, which could be useful in outbreaks or situations where leptospirosis is endemic.
Keywords: Leptospira; adjuvant; adjuvants; cattle; immune response; vaccine; vaccines; veterinary vaccine development.
Figures
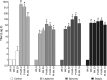
Similar articles
-
Bovine immune response to leptospira antigen in different novel adjuvants and vaccine delivery platforms.Vaccine. 2020 Apr 16;38(18):3464-3473. doi: 10.1016/j.vaccine.2020.02.086. Epub 2020 Mar 20. Vaccine. 2020. PMID: 32204939
-
Efficacy of a flexible schedule for administration of a Leptospira borgpetersenii serovar Hardjo bacterin to beef calves.Am J Vet Res. 2014 May;75(5):507-12. doi: 10.2460/ajvr.75.5.507. Am J Vet Res. 2014. PMID: 24762025 Clinical Trial.
-
A Leptospira borgpetersenii serovar Hardjo vaccine induces a Th1 response, activates NK cells, and reduces renal colonization.Clin Vaccine Immunol. 2011 Apr;18(4):684-91. doi: 10.1128/CVI.00288-10. Epub 2011 Feb 2. Clin Vaccine Immunol. 2011. PMID: 21288995 Free PMC article.
-
Meta-analysis of the efficacy of Leptospira serovar Hardjo vaccines to prevent urinary shedding in cattle.Prev Vet Med. 2018 May 1;153:71-76. doi: 10.1016/j.prevetmed.2018.02.015. Epub 2018 Mar 1. Prev Vet Med. 2018. PMID: 29653738
-
Adjuvanted leptospiral vaccines: Challenges and future development of new leptospirosis vaccines.Vaccine. 2019 Jul 9;37(30):3961-3973. doi: 10.1016/j.vaccine.2019.05.087. Epub 2019 Jun 8. Vaccine. 2019. PMID: 31186193 Review.
Cited by
-
Prevalence of Anti-Leptospira Antibodies in Bovines (2018-2023) and Reported Serovars and Serogroups-A Systematic Review With Meta-Analysis.Vet Med Sci. 2025 Sep;11(5):e70529. doi: 10.1002/vms3.70529. Vet Med Sci. 2025. PMID: 40728080 Free PMC article.
-
Leptospira borgpetersenii Leucine-Rich Repeat Proteins Provide Strong Protective Efficacy as Novel Leptospiral Vaccine Candidates.Trop Med Infect Dis. 2022 Dec 22;8(1):6. doi: 10.3390/tropicalmed8010006. Trop Med Infect Dis. 2022. PMID: 36668913 Free PMC article.
-
Comparison of the PF07598-Encoded Virulence-Modifying Proteins of L. interrogans and L. borgpetersenii.Trop Med Infect Dis. 2022 Dec 26;8(1):14. doi: 10.3390/tropicalmed8010014. Trop Med Infect Dis. 2022. PMID: 36668921 Free PMC article. Review.
-
Prevalence and risk factors of bovine leptospirosis in the Ecuadorian Amazon.Vet World. 2024 Nov;17(11):2612-2618. doi: 10.14202/vetworld.2024.2612-2618. Epub 2024 Nov 25. Vet World. 2024. PMID: 39829649 Free PMC article.
References
-
- Haake DA, Levett PN. 2015. Leptospirosis in humans, p 65–97. In Adler B (ed), Leptospira and leptospirosis. Springer Berlin Heidelberg, Berlin, Heidelberg.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
