Endocrine complications of immunotherapies: a review
- PMID: 33762389
- PMCID: PMC8002767
- DOI: 10.7861/clinmed.2020-0827
Endocrine complications of immunotherapies: a review
Abstract
Use of immune checkpoint inhibitors in cancer treatment has increased vastly over the past decade, as both single and combination agent therapies. While having a positive impact on survival rates, adverse effects have been noted, with endocrine effects in around 10% of patients. Thyroid disease and hypophysitis are the most commonly encountered, with diabetes mellitus and primary adrenal insufficiency also reported, as well as more rare endocrinopathies. Patient and clinician education to raise awareness of these effects, as well as regular monitoring to enable early recognition, diagnosis and prompt treatment of the immune side effects, are key. In this review, we discuss the aetiology, presentation and management of the endocrine complications of immunotherapies that are relevant to the general physician, as well as highlighting important areas where further research is still needed.
Keywords: cancer; endocrine; hypophysitis; immunotherapy; thyroid.
© Royal College of Physicians 2021. All rights reserved.
Figures

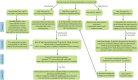
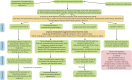
References
-
- Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 2019;16:563–80. - PubMed
-
- Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29(Suppl 4):iv264–6. - PubMed
-
- Castinetti F, Borson-Chazot F. Introduction to expert opinion on endocrine complications of new anticancer therapies. Ann Endocrinol (Paris) 2018;79:535–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

