Newcastle Disease Virus Entry into Chicken Macrophages via a pH-Dependent, Dynamin and Caveola-Mediated Endocytic Pathway That Requires Rab5
- PMID: 33762417
- PMCID: PMC8437353
- DOI: 10.1128/JVI.02288-20
Newcastle Disease Virus Entry into Chicken Macrophages via a pH-Dependent, Dynamin and Caveola-Mediated Endocytic Pathway That Requires Rab5
Abstract
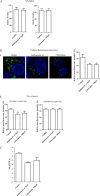
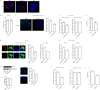
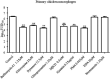
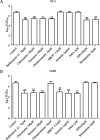
The cellular entry pathways and the mechanisms of Newcastle disease virus (NDV) entry into cells are poorly characterized. In this study, we demonstrated that chicken interferon-induced transmembrane protein 1 (chIFITM1), which is located in the early endosomes, could limit the replication of NDV in chicken macrophage cell line HD11, suggesting the endocytic entry of NDV into chicken macrophages. Then, we presented a systematic study about the entry mechanism of NDV into chicken macrophages. First, we demonstrated that a low-pH condition and dynamin were required during NDV entry. However, NDV entry into chicken macrophages was independent of clathrin-mediated endocytosis. We also found that NDV entry was dependent on membrane cholesterol. The NDV entry and replication were significantly reduced by nystatin and phorbol 12-myristate 13-acetate treatment, overexpression of dominant-negative (DN) caveolin-1, or knockdown of caveolin-1, suggesting that NDV entry depends on caveola-mediated endocytosis. However, macropinocytosis did not play a role in NDV entry into chicken macrophages. In addition, we found that Rab5, rather than Rab7, was involved in the entry and traffic of NDV. The colocalization of NDV with Rab5 and early endosome suggested that NDV virion was transported to early endosomes in a Rab5-dependent manner after internalization. Of particular note, the caveola-mediated endocytosis was also utilized by NDV to enter primary chicken macrophages. Moreover, NDV entered different cell types using different pathways. Collectively, our findings demonstrate for the first time that NDV virion enters chicken macrophages via a pH-dependent, dynamin and caveola-mediated endocytosis pathway and that Rab5 is involved in the traffic and location of NDV. IMPORTANCE Although the pathogenesis of Newcastle disease virus (NDV) has been extensively studied, the detailed mechanism of NDV entry into host cells is largely unknown. Macrophages are the first-line defenders of host defense against infection of pathogens. Chicken macrophages are considered one of the main types of target cells during NDV infection. Here, we comprehensively investigated the entry mechanism of NDV in chicken macrophages. This is the first report to demonstrate that NDV enters chicken macrophages via a pH-dependent, dynamin and caveola-mediated endocytosis pathway that requires Rab5. The result is important for our understanding of the entry of NDV in chicken macrophages, which will further advance the knowledge of NDV pathogenesis and provide useful clues for the development of novel preventive or therapeutic strategies against NDV infection. In addition, this information will contribute to our further understanding of pathogenesis with regard to other members of the Avulavirus genus in the Paramyxoviridae family.
Keywords: Newcastle disease virus; Rab5; chicken macrophage; endocytic pathway.
Figures





References
-
- Alexander DJ. 2009. Ecology and epidemiology of Newcastle disease, p 19–26. In Capua I, Alexander DJ (ed), Avian influenza and Newcastle disease. Springer, New York, NY.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

