Short-term outcomes of intravitreal brolucizumab for treatment-naïve neovascular age-related macular degeneration with type 1 choroidal neovascularization including polypoidal choroidal vasculopathy
- PMID: 33762600
- PMCID: PMC7990919
- DOI: 10.1038/s41598-021-86014-7
Short-term outcomes of intravitreal brolucizumab for treatment-naïve neovascular age-related macular degeneration with type 1 choroidal neovascularization including polypoidal choroidal vasculopathy
Abstract
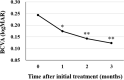
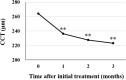
We evaluated the efficacy and safety of loading phase treatment with intravitreal brolucizumab for neovascular age-related macular degeneration (nAMD) with type 1 choroidal neovascularization (CNV). We analyzed consecutive 42 eyes of 40 patients with treatment-naïve nAMD associated with type 1 CNV. Three monthly injections of brolucizumab were completed in 36 eyes (85.7%). In those cases, best-corrected visual acuity (BCVA) was 0.24 ± 0.27 at baseline and improved significantly to 0.12 ± 0.23 after 3 months (P < 0.001). Central macular thickness was 301 ± 110 µm at baseline and decreased significantly to 160 ± 49 µm after 3 months (P < 0.001). Dry macula was achieved in 34 eyes (94.4%) after the loading phase. Central choroidal thickness was 264 ± 89 µm at baseline and decreased significantly to 223 ± 81 µm after 3 months (P < 0.001). Indocyanine green angiography after the loading phase revealed complete regression of polypoidal lesions in 15 of the 19 eyes (78.9%) with polypoidal lesions. Non-infectious intraocular inflammation (IOI) was observed in 8 of 42 eyes (19.0%) during the loading phase, while showing amelioration in response to combination therapy with topical and subtenon injection of steroids. In these eyes, BCVA after 3 months had not deteriorated as compared to that at baseline. These results indicate that loading phase treatment with intravitreal brolucizumab might be effective for improving visual acuity and reducing exudative changes in eyes with nAMD associated with type 1 CNV. Moreover, polypoidal lesions appear to frequently regress after this treatment. However, we must monitor patients carefully for brolucizumab-related IOI, and administer steroid therapy promptly.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
One-year results of treat-and-extend regimen with intravitreal brolucizumab for treatment-naïve neovascular age-related macular degeneration with type 1 macular neovascularization.Sci Rep. 2022 May 17;12(1):8195. doi: 10.1038/s41598-022-10578-1. Sci Rep. 2022. PMID: 35581196 Free PMC article.
-
Long-term efficacy and safety of brolucizumab in neovascular age-related macular degeneration: A multicentre retrospective real-world study.Acta Ophthalmol. 2024 Nov;102(7):e1018-e1028. doi: 10.1111/aos.16699. Epub 2024 May 5. Acta Ophthalmol. 2024. PMID: 38706195
-
Intravitreal Aflibercept versus Brolucizumab for Treatment-Naive Neovascular Age-Related Macular Degeneration with Type 1 Macular Neovascularization: Comparison of Short-Term Outcomes.Ophthalmologica. 2022;245(5):413-420. doi: 10.1159/000526044. Epub 2022 Jul 14. Ophthalmologica. 2022. PMID: 35834995
-
Usage of brolucizumab as treatment for wet age-related macular degeneration (AMD) and polypoidal choroidal vasculopathy (PCV): A narrative review.Medicine (Baltimore). 2025 Jun 6;104(23):e42666. doi: 10.1097/MD.0000000000042666. Medicine (Baltimore). 2025. PMID: 40489855 Free PMC article. Review.
-
Brolucizumab: Evolution through Preclinical and Clinical Studies and the Implications for the Management of Neovascular Age-Related Macular Degeneration.Ophthalmology. 2020 Jul;127(7):963-976. doi: 10.1016/j.ophtha.2019.12.031. Epub 2020 Jan 17. Ophthalmology. 2020. PMID: 32107066 Review.
Cited by
-
Association of Polyp Regression after Loading Phase with 12-Month Outcomes of Eyes with Polypoidal Choroidal Vasculopathy.Pharmaceuticals (Basel). 2024 May 27;17(6):687. doi: 10.3390/ph17060687. Pharmaceuticals (Basel). 2024. PMID: 38931354 Free PMC article.
-
One-year outcome of brolucizumab for neovascular age-related macular degeneration in Japanese patients.Sci Rep. 2024 Jan 30;14(1):2451. doi: 10.1038/s41598-024-52747-4. Sci Rep. 2024. PMID: 38291120 Free PMC article.
-
One-year outcomes of intravitreal brolucizumab injections in patients with polypoidal choroidal vasculopathy.Sci Rep. 2022 May 14;12(1):7987. doi: 10.1038/s41598-022-12216-2. Sci Rep. 2022. PMID: 35568780 Free PMC article.
-
Brolucizumab for the Treatment of Degenerative Macular Conditions: A Review of Clinical Studies.Drug Des Devel Ther. 2022 Aug 9;16:2659-2680. doi: 10.2147/DDDT.S378450. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 35971530 Free PMC article. Review.
-
Comparison of Outcomes between 3 Monthly Brolucizumab and Aflibercept Injections for Polypoidal Choroidal Vasculopathy.Biomedicines. 2021 Sep 5;9(9):1164. doi: 10.3390/biomedicines9091164. Biomedicines. 2021. PMID: 34572350 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

