Potential novel biomarkers for chronic lung allograft dysfunction and azithromycin responsive allograft dysfunction
- PMID: 33762606
- PMCID: PMC7990920
- DOI: 10.1038/s41598-021-85949-1
Potential novel biomarkers for chronic lung allograft dysfunction and azithromycin responsive allograft dysfunction
Abstract
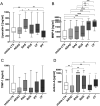
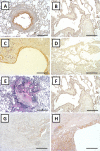
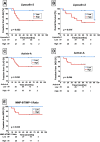
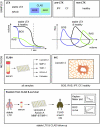
Chronic Lung Allograft Dysfunction (CLAD), manifesting as Bronchiolitis Obliterans Syndrome (BOS) or Restrictive Allograft Syndrome (RAS), is the main reason for adverse long-term outcome after Lung Transplantation (LTX). Until now, no specific biomarkers exist to differentiate between CLAD phenotypes. Therefore, we sought to find suitable cytokines to distinguish between BOS, RAS and Azithromycin Responsive Allograft Dysfunction (ARAD); and reveal potential similarities or differences to end-stage fibrotic diseases. We observed significantly increased Lipocalin-2 serum concentrations in RAS compared to BOS patients. In addition, in RAS patients immunohistochemistry revealed Lipocalin-2 expression in bronchial epithelium and alveolar walls. Patients with ARAD showed significantly lower Activin-A serum concentrations compared to Stable-LTX and BOS patients. Further, increased serum concentrations of Lipocalin-2 and Activin-A were predictors of worse freedom-from-CLAD in Stable-LTX patients. These biomarkers serve as promising serum biomarkers for CLAD prediction and seem suitable for implementation in clinical practice.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

