Risk compensation after HIV-1 vaccination may accelerate viral adaptation and reduce cost-effectiveness: a modeling study
- PMID: 33762616
- PMCID: PMC7991033
- DOI: 10.1038/s41598-021-85487-w
Risk compensation after HIV-1 vaccination may accelerate viral adaptation and reduce cost-effectiveness: a modeling study
Abstract
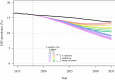
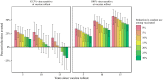
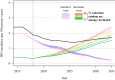
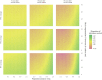
Pathogen populations can evolve in response to selective pressure from vaccine-induced immune responses. For HIV, models predict that viral adaptation, either via strain replacement or selection on de novo mutation, may rapidly reduce the effectiveness of an HIV vaccine. We hypothesized that behavioral risk compensation after vaccination may accelerate the transmission of vaccine resistant strains, increasing the rate of viral adaptation and leading to a more rapid decline in vaccine effectiveness. To test our hypothesis, we modeled: (a) the impact of risk compensation on rates of HIV adaptation via strain replacement in response to a partially effective vaccine; and (b) the combined impact of risk compensation and viral adaptation on vaccine-mediated epidemic control. We used an agent-based epidemic model that was calibrated to HIV-1 trends in South Africa, and includes demographics, sexual network structure and behavior, and within-host disease dynamics. Our model predicts that risk compensation can increase the rate of HIV viral adaptation in response to a vaccine. In combination, risk compensation and viral adaptation can, under certain scenarios, reverse initial declines in prevalence due to vaccination, and result in HIV prevalence at 15 years equal to or greater than prevalence without a vaccine.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
HIV population-level adaptation can rapidly diminish the impact of a partially effective vaccine.Vaccine. 2018 Jan 25;36(4):514-520. doi: 10.1016/j.vaccine.2017.12.004. Epub 2017 Dec 11. Vaccine. 2018. PMID: 29241646 Free PMC article.
-
Targeting and vaccine durability are key for population-level impact and cost-effectiveness of a pox-protein HIV vaccine regimen in South Africa.Vaccine. 2019 Apr 10;37(16):2258-2267. doi: 10.1016/j.vaccine.2019.02.073. Epub 2019 Mar 16. Vaccine. 2019. PMID: 30890385 Free PMC article.
-
The potential impact of RV144-like vaccines in rural South Africa: a study using the STDSIM microsimulation model.Vaccine. 2011 Aug 18;29(36):6100-6. doi: 10.1016/j.vaccine.2011.06.059. Epub 2011 Jun 22. Vaccine. 2011. PMID: 21703321 Free PMC article.
-
[HIV-1 vaccination--is there hope?].Ther Umsch. 2005 Oct;62(10):695-702. doi: 10.1024/0040-5930.62.10.695. Ther Umsch. 2005. PMID: 16277037 Review. German.
-
Advances in HIV-1 Vaccine Development.Viruses. 2018 Apr 1;10(4):167. doi: 10.3390/v10040167. Viruses. 2018. PMID: 29614779 Free PMC article. Review.
Cited by
-
Evolution of HIV virulence in response to disease-modifying vaccines: A modeling study.Vaccine. 2023 Oct 13;41(43):6461-6469. doi: 10.1016/j.vaccine.2023.08.071. Epub 2023 Sep 14. Vaccine. 2023. PMID: 37714749 Free PMC article.
-
Association of HCV Prior Infection and Unprotected Sex on Subsequent HIV Acquisition Risk in the Era of Treatment as Prevention.Front Med (Lausanne). 2022 May 24;9:902271. doi: 10.3389/fmed.2022.902271. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35685415 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

