Conventional versus reverse sequence of neoadjuvant epirubicin/cyclophosphamide and docetaxel: sequencing results from ABCSG-34
- PMID: 33762716
- PMCID: PMC8144560
- DOI: 10.1038/s41416-021-01284-2
Conventional versus reverse sequence of neoadjuvant epirubicin/cyclophosphamide and docetaxel: sequencing results from ABCSG-34
Abstract
Background: Preoperative chemotherapy containing anthracyclines and taxanes is well established in early-stage breast cancer. Previous studies have suggested that the chemotherapy sequence may matter but definitive evidence is missing. ABCSG trial 34 evaluated the activity of the MUC1 vaccine tecemotide when added to neoadjuvant treatment; the study provided the opportunity for the second randomisation to compare two different anthracycline/taxane sequences.
Methods: HER2-negative early-stage breast cancer patients were recruited to this randomised multicentre Phase 2 study. Patients in the chemotherapy cohort (n = 311) were additionally randomised to a conventional or reversed sequence of epirubicin/cyclophosphamide and docetaxel. Residual cancer burden (RCB) with/without tecemotide was defined as primary study endpoint; RCB in the two chemotherapy groups was a key secondary endpoint.
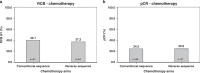
Results: No significant differences in terms of RCB 0/I (40.1% vs. 37.2%; P = 0.61) or pathologic complete response (pCR) rates (24.3% vs. 25%, P = 0.89) were observed between conventional or reverse chemotherapy sequence. No new safety signals were reported, and upfront docetaxel did not result in decreased rates of treatment delay or discontinuation.
Conclusion: Upfront docetaxel did not improve chemotherapy activity or tolerability; these results suggest that upfront neoadjuvant treatment with anthracyclines remains a valid option.
Conflict of interest statement
R.B. reports advisory roles at AstraZeneca, Celgene, Daiichi, Eisai, Eli-Lilly, MSD, Novartis, Pfizer, Pierre-Fabre, Puma, Roche, Samsung, lecture honoraria: Accord, AstraZeneca, BMS, Celgene, Eli-Lilly, Novartis, Pfizer, Pierre-Fabre, Roche, Sandoz and received research support from Daiichi, Novartis and Roche, all outside of the submitted work. M.H. reports honoraria and/or travel support from Amgen, AstraZeneca, Celgene, Eli-Lilly, Pfizer, Novartis, Roche all outside the submitted work. A.L.P. reports honoraria and/or travel support from Abbvie, Amgen, AstraZeneca, Bayer, Celgene, Gilead, Janssen, Eli-Lilly, MSD, Pfizer, Novartis, Roche, Sanofi and Takeda all outside the submitted work. D.E. reports honoraria/travel support from Amgen, AstraZeneca, Celgene, Eli-Lilly, MSD, Novartis, Pfizer, Roche and Myriad all outside of the submitted work. M.F. has received honoraria for advisory boards from AstraZeneca, Bayer, Biomedica, Boehringer Ingelheim, Eli-Lilly, Merck Sharp & Dohme, Myriad Genetics Inc., Pfizer and Roche. M.G. reports personal fees/travel support from Amgen, AstraZeneca, Celgene, Eli-Lilly, Invectys, Pfizer, Novartis, Puma, Nanostring, Roche, Medison, LifeBrain, all outside the submitted work; an immediate family member is employed by Sandoz. Z.B.-H. has advisory roles at Biomedica, Roche and Novartis, received lecture honoraria and travel support from Roche and research support from Boehringer Ingelheim. C.F.S., G.P., H.S., A.P., E.P., V.B.-R., R.G., M.R., M.K.T., V.W., P.S., P.D., F.F., R.E., R.J., M.B., C.T. and S.F. declare no competing interests.
Figures
References
-
- Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, Bonnefoi H, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann. Oncol. 2007;18:1927–1934. doi: 10.1093/annonc/mdm201. - DOI - PubMed
-
- Bear, H. D., Anderson, S., Smith, R. E., Geyer, C. E. Jr., Mamounas, E. P., Fisher, B. et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J. Clin. Oncol.24, 2019–2027 (1999). - PubMed
-
- von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

