Presymptomatic training mitigates functional deficits in a mouse model of Rett syndrome
- PMID: 33762729
- PMCID: PMC8093094
- DOI: 10.1038/s41586-021-03369-7
Presymptomatic training mitigates functional deficits in a mouse model of Rett syndrome
Abstract
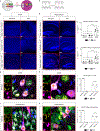
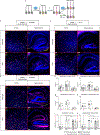
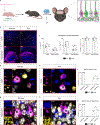
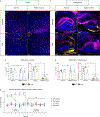
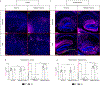
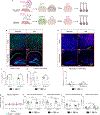
Mutations in the X-linked gene MECP2 cause Rett syndrome, a progressive neurological disorder in which children develop normally for the first one or two years of life before experiencing profound motor and cognitive decline1-3. At present there are no effective treatments for Rett syndrome, but we hypothesized that using the period of normal development to strengthen motor and memory skills might confer some benefit. Here we find, using a mouse model of Rett syndrome, that intensive training beginning in the presymptomatic period dramatically improves the performance of specific motor and memory tasks, and significantly delays the onset of symptoms. These benefits are not observed when the training begins after symptom onset. Markers of neuronal activity and chemogenetic manipulation reveal that task-specific neurons that are repeatedly activated during training develop more dendritic arbors and have better neurophysiological responses than those in untrained animals, thereby enhancing their functionality and delaying symptom onset. These results provide a rationale for genetic screening of newborns for Rett syndrome, as presymptomatic intervention might mitigate symptoms or delay their onset. Similar strategies should be studied for other childhood neurological disorders.
Figures





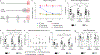
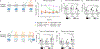
References
-
- Amir RE et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23, 185–188 (1999). - PubMed
-
- Hagburg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. Ann Neurol 14, 471–479 (1983). - PubMed
-
- Sandweiss AJ, Brandt VL, Zoghbi HY Advances in understanding of Rett syndrome and MECP2 duplication syndrome: prospects for future therapies. Lancet Neurol 19, 689–698 (2020). - PubMed
-
- Laurvick CL et al. Rett syndrome in Australia: a review of the epidemiology. J Pediatr 148, 347–353 (2006). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

