A vaccine targeting mutant IDH1 in newly diagnosed glioma
- PMID: 33762734
- PMCID: PMC8046668
- DOI: 10.1038/s41586-021-03363-z
A vaccine targeting mutant IDH1 in newly diagnosed glioma
Abstract
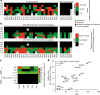
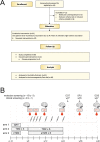
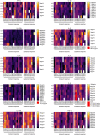
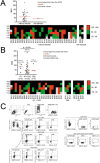
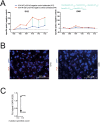
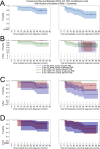
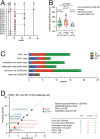
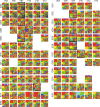
Mutated isocitrate dehydrogenase 1 (IDH1) defines a molecularly distinct subtype of diffuse glioma1-3. The most common IDH1 mutation in gliomas affects codon 132 and encodes IDH1(R132H), which harbours a shared clonal neoepitope that is presented on major histocompatibility complex (MHC) class II4,5. An IDH1(R132H)-specific peptide vaccine (IDH1-vac) induces specific therapeutic T helper cell responses that are effective against IDH1(R132H)+ tumours in syngeneic MHC-humanized mice4,6-8. Here we describe a multicentre, single-arm, open-label, first-in-humans phase I trial that we carried out in 33 patients with newly diagnosed World Health Organization grade 3 and 4 IDH1(R132H)+ astrocytomas (Neurooncology Working Group of the German Cancer Society trial 16 (NOA16), ClinicalTrials.gov identifier NCT02454634). The trial met its primary safety endpoint, with vaccine-related adverse events restricted to grade 1. Vaccine-induced immune responses were observed in 93.3% of patients across multiple MHC alleles. Three-year progression-free and death-free rates were 0.63 and 0.84, respectively. Patients with immune responses showed a two-year progression-free rate of 0.82. Two patients without an immune response showed tumour progression within two years of first diagnosis. A mutation-specificity score that incorporates the duration and level of vaccine-induced IDH1(R132H)-specific T cell responses was associated with intratumoral presentation of the IDH1(R132H) neoantigen in pre-treatment tumour tissue. There was a high frequency of pseudoprogression, which indicates intratumoral inflammatory reactions. Pseudoprogression was associated with increased vaccine-induced peripheral T cell responses. Combined single-cell RNA and T cell receptor sequencing showed that tumour-infiltrating CD40LG+ and CXCL13+ T helper cell clusters in a patient with pseudoprogression were dominated by a single IDH1(R132H)-reactive T cell receptor.
Conflict of interest statement
M.P., T.B. and W.W. are inventors and patent-holders on ‘Peptides for use in treating or diagnosing IDH1R132H positive cancers’ (EP2800580B1).
Figures







Comment in
-
IDH1 vaccine shows potential in astrocytoma.Nat Rev Neurol. 2021 May;17(5):262. doi: 10.1038/s41582-021-00495-8. Nat Rev Neurol. 2021. PMID: 33846618 No abstract available.
-
A vaccine for glioma.Nat Cancer. 2021 Jun;2(6):584-586. doi: 10.1038/s43018-021-00228-2. Nat Cancer. 2021. PMID: 35121939 No abstract available.
-
Vaccination for IDH-mutant tumors: A novel therapeutic approach applied to glioma.Med. 2021 May 14;2(5):450-452. doi: 10.1016/j.medj.2021.04.021. Med. 2021. PMID: 35590222
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

