Transcutaneous Auricular Neurostimulation (tAN): A Novel Adjuvant Treatment in Neonatal Opioid Withdrawal Syndrome
- PMID: 33762918
- PMCID: PMC7982745
- DOI: 10.3389/fnhum.2021.648556
Transcutaneous Auricular Neurostimulation (tAN): A Novel Adjuvant Treatment in Neonatal Opioid Withdrawal Syndrome
Abstract
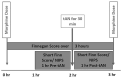
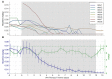
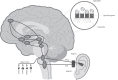
Maternal opioid use during pregnancy is a growing national problem and can lead to newborns developing neonatal opioid withdrawal syndrome (NOWS) soon after birth. Recent data demonstrates that nearly every 15 min a baby is born in the United States suffering from NOWS. The primary treatment for NOWS is opioid replacement therapy, commonly oral morphine, which has neurotoxic effects on the developing brain. There is an urgent need for non-opioid treatments for NOWS. Transcutaneous auricular neurostimulation (tAN), a novel and non-invasive form of electrostimulation, may serve as a promising alternative to morphine. tAN is delivered via a multichannel earpiece electrode worn on and around the left ear, targeting two cranial nerves-the vagus and trigeminal nerves. Prior research suggests that auricular neurostimulation exerts an anxiolytic effect on the body by releasing endogenous opioids and reduces withdrawal symptoms in adults actively withdrawing from opioids. In this first-in-human prospective, open-label trial, we investigated tAN as an adjuvant to morphine therapy in eight infants >33 weeks gestational age suffering from NOWS and receiving oral morphine treatment. Infants received tAN for 30 min 1 h before receiving a morphine dose. tAN was delivered at 0.1 mA below perception intensity at two different nerve targets on the ear: Region 1, the auricular branch of the vagus nerve; and Region 2, the auriculotemporal nerve. tAN was delivered up to four times daily for a maximum of 12 days. The primary outcome measures were safety [heart rate monitoring, Neonatal Infant Pain Scale (NIPS), and skin irritation] and morphine length of treatment (LOT). tAN was well-tolerated and resulted in no unanticipated adverse events. Comparing to the national average of 23 days, the average oral morphine LOT was 13.3 days (median 9 days) and the average LOT after tAN initiation was 7 days (median 6 days). These preliminary data suggest that tAN is safe and may serve as a promising alternative adjuvant for treating NOWS and reducing the amount of time an infant receives oral morphine.
Keywords: bioelectronic medicine; morphine; neonatal opioid withdrawal syndrome (NOWS); non-invasive neuromodulation; opioids; tAN; transcutaneous auricular neurostimulation.
Copyright © 2021 Jenkins, Khodaparast, O’Leary, Washburn, Covalin and Badran.
Conflict of interest statement
BB and DJ are named inventors on brain stimulation patents/devices assigned to the Medical University of South Carolina. BB has equity in Bodhi NeuroTech, Inc. NK, SW, and AC are employees and shareholders of Spark Biomedical, Inc. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Delivering transcutaneous auricular neurostimulation (tAN) to improve symptoms associated with opioid withdrawal: results from a prospective clinical trial.Bioelectron Med. 2022 Aug 18;8(1):12. doi: 10.1186/s42234-022-00095-x. Bioelectron Med. 2022. PMID: 35978394 Free PMC article.
-
Opioid Treatment for Neonatal Opioid Withdrawal Syndrome: Current Challenges and Future Approaches.J Clin Pharmacol. 2021 Jul;61(7):857-870. doi: 10.1002/jcph.1811. Epub 2021 Feb 1. J Clin Pharmacol. 2021. PMID: 33382111 Review.
-
Pragmatic, randomized, blinded trial to shorten pharmacologic treatment of newborns with neonatal opioid withdrawal syndrome (NOWS).Trials. 2023 Jul 21;24(1):466. doi: 10.1186/s13063-023-07378-x. Trials. 2023. PMID: 37480087 Free PMC article. Clinical Trial.
-
Gabapentin as Adjunctive Therapy in Neonatal Opioid Withdrawal Syndrome: A Case Series.J Pediatr Pharmacol Ther. 2023;28(4):368-373. doi: 10.5863/1551-6776-28.4.368. Epub 2023 Aug 9. J Pediatr Pharmacol Ther. 2023. PMID: 37795276 Free PMC article.
-
Clinical care of neonates undergoing opioid withdrawal in the immediate postpartum period.Neurotoxicol Teratol. 2021 Jul-Aug;86:106978. doi: 10.1016/j.ntt.2021.106978. Epub 2021 Apr 7. Neurotoxicol Teratol. 2021. PMID: 33838247 Review.
Cited by
-
Acupuncture for neonatal abstinence syndrome in newborn infants.Cochrane Database Syst Rev. 2025 Feb 21;2(2):CD014160. doi: 10.1002/14651858.CD014160.pub2. Cochrane Database Syst Rev. 2025. PMID: 39981752
-
Noninvasive Vagal Nerve Stimulation for Opioid Use Disorder.Ann Depress Anxiety. 2023;10(1):1117. Epub 2023 Aug 2. Ann Depress Anxiety. 2023. PMID: 38074313 Free PMC article.
-
Transcutaneous auricular Vagus Nerve Stimulation and Median Nerve Stimulation reduce acute stress in young healthy adults: a single-blind sham-controlled crossover study.Front Neurosci. 2023 Sep 7;17:1213982. doi: 10.3389/fnins.2023.1213982. eCollection 2023. Front Neurosci. 2023. PMID: 37746156 Free PMC article.
-
Electrical stimulation of the trigeminal nerve improves olfaction in healthy individuals: A randomized, double-blind, sham-controlled trial.Brain Stimul. 2022 May-Jun;15(3):761-768. doi: 10.1016/j.brs.2022.05.005. Epub 2022 May 11. Brain Stimul. 2022. PMID: 35561963 Free PMC article. Clinical Trial.
-
The Future Is Noninvasive: A Brief Review of the Evolution and Clinical Utility of Vagus Nerve Stimulation.Focus (Am Psychiatr Publ). 2022 Jan;20(1):3-7. doi: 10.1176/appi.focus.20210023. Epub 2022 Jan 25. Focus (Am Psychiatr Publ). 2022. PMID: 35746934 Free PMC article. Review.
References
-
- Badran B. W., Brown J. C., Dowdle L. T., Mithoefer O. J., LaBate N. T., Coatsworth J., et al. . (2018a). Tragus or cymba conchae? Investigating the anatomical foundation of transcutaneous auricular vagus nerve stimulation (taVNS). Brain Stimul. 11, 947–948. 10.1016/j.brs.2018.06.003 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

