Mitochondrial Function, Fatty Acid Metabolism, and Body Composition in the Hyperbilirubinemic Gunn Rat
- PMID: 33762933
- PMCID: PMC7982585
- DOI: 10.3389/fphar.2021.586715
Mitochondrial Function, Fatty Acid Metabolism, and Body Composition in the Hyperbilirubinemic Gunn Rat
Abstract
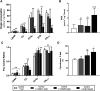
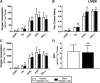
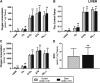
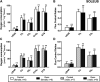
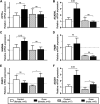
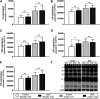
Background: Circulating bilirubin is associated with reduced adiposity in human and animal studies. A possible explanation is provided by in vitro data that demonstrates that bilirubin inhibits mitochondrial function and decreases efficient energy production. However, it remains unclear whether hyperbilirubinemic animals have similar perturbed mitochondrial function and whether this is important for regulation of energy homeostasis. Aim: To investigate the impact of unconjugated hyperbilirubinemia on body composition, and mitochondrial function in hepatic tissue and skeletal muscle. Materials and Methods: 1) Food intake and bodyweight gain of 14-week old hyperbilirubinemic Gunn (n = 19) and normobilirubinemic littermate (control; n = 19) rats were measured over a 17-day period. 2) Body composition was determined using dual-energy X-ray absorptiometry and by measuring organ and skeletal muscle masses. 3) Mitochondrial function was assessed using high-resolution respirometry of homogenized liver and intact permeabilized extensor digitorum longus and soleus fibers. 4) Liver tissue was flash frozen for later gene (qPCR), protein (Western Blot and citrate synthase activity) and lipid analysis. Results: Female hyperbilirubinemic rats had significantly reduced fat mass (Gunn: 9.94 ± 5.35 vs. Control: 16.6 ± 6.90 g, p < 0.05) and hepatic triglyceride concentration (Gunn: 2.39 ± 0.92 vs. Control: 4.65 ± 1.67 mg g-1, p < 0.01) compared to normobilirubinemic controls. Furthermore, hyperbilirubinemic rats consumed fewer calories daily (p < 0.01) and were less energetically efficient (Gunn: 8.09 ± 5.75 vs. Control: 14.9 ± 5.10 g bodyweight kcal-1, p < 0.05). Hepatic mitochondria of hyperbilirubinemic rats demonstrated increased flux control ratio (FCR) via complex I and II (CI+II) (Gunn: 0.78 ± 0.16 vs. Control: 0.62 ± 0.09, p < 0.05). Similarly, exogenous addition of 31.3 or 62.5 μM unconjugated bilirubin to control liver homogenates significantly increased CI+II FCR (p < 0.05). Hepatic PGC-1α gene expression was significantly increased in hyperbilirubinemic females while FGF21 and ACOX1 was significantly greater in male hyperbilirubinemic rats (p < 0.05). Finally, hepatic mitochondrial complex IV subunit 1 protein expression was significantly increased in female hyperbilirubinemic rats (p < 0.01). Conclusions: This is the first study to comprehensively assess body composition, fat metabolism, and mitochondrial function in hyperbilirubinemic rats. Our findings show that hyperbilirubinemia is associated with reduced fat mass, and increased hepatic mitochondrial biogenesis, specifically in female animals, suggesting a dual role of elevated bilirubin and reduced UGT1A1 function on adiposity and body composition.
Keywords: Gunn rat; hyperbilirubinemia; lipids; metabolism; mitochondria; respiration; triglycerides; unconjugated bilirubin (UCB).
Copyright © 2021 Vidimce, Pillay, Shrestha, Dong, Neuzil, Wagner, Holland and Bulmer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor declared a past co-authorship with one of the authors AB.
Figures



References
-
- Boon A. C., Hawkins C. L., Bisht K., Coombes J. S., Bakrania B., Wagner K. H., et al. (2012). Reduced circulating oxidized LDL is associated with hypocholesterolemia and enhanced thiol status in Gilbert syndrome. Free Radic. Biol. Med. 52, 2120–2127. 10.1016/j.freeradbiomed.2012.03.002 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

