Simplified Dosing Regimens for Gentamicin in Neonatal Sepsis
- PMID: 33762945
- PMCID: PMC7982486
- DOI: 10.3389/fphar.2021.624662
Simplified Dosing Regimens for Gentamicin in Neonatal Sepsis
Abstract
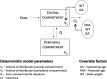
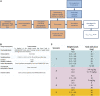
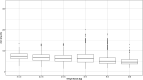
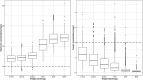
Background: The effectiveness of antibiotics for the treatment of severe bacterial infections in newborns in resource-limited settings has been determined by empirical evidence. However, such an approach does not warrant optimal exposure to antibiotic agents, which are known to show different disposition characteristics in this population. Here we evaluate the rationale for a simplified regimen of gentamicin taking into account the effect of body size and organ maturation on pharmacokinetics. The analysis is supported by efficacy data from a series of clinical trials in this population. Methods: A previously published pharmacokinetic model was used to simulate gentamicin concentration vs. time profiles in a virtual cohort of neonates. Model predictive performance was assessed by supplementary external validation procedures using therapeutic drug monitoring data collected in neonates and young infants with or without sepsis. Subsequently, clinical trial simulations were performed to characterize the exposure to intra-muscular gentamicin after a q.d. regimen. The selection of a simplified regimen was based on peak and trough drug levels during the course of treatment. Results: In contrast to current World Health Organization guidelines, which recommend gentamicin doses between 5 and 7.5 mg/kg, our analysis shows that gentamicin can be used as a fixed dose regimen according to three weight-bands: 10 mg for patients with body weight <2.5 kg, 16 mg for patients with body weight between 2.5 and 4 kg, and 30 mg for those with body weight >4 kg. Conclusion: The choice of the dose of an antibiotic must be supported by a strong scientific rationale, taking into account the differences in drug disposition in the target patient population. Our analysis reveals that a simplified regimen is feasible and could be used in resource-limited settings for the treatment of sepsis in neonates and young infants with sepsis aged 0-59 days.
Keywords: bacterial infection; dosing optimization; gentamicin; modeling and simulation; neonatal sepsis; pharmacokinetics; resource-limited and remote setting.
Copyright © 2021 D’Agate, Musuamba, Jacqz-Aigrain and Della Pasqua.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Dose Rationale for Amoxicillin in Neonatal Sepsis When Referral Is Not Possible.Front Pharmacol. 2020 Sep 25;11:521933. doi: 10.3389/fphar.2020.521933. eCollection 2020. Front Pharmacol. 2020. PMID: 33117151 Free PMC article.
-
Community-based antibiotic delivery for possible serious bacterial infections in neonates in low- and middle-income countries.Cochrane Database Syst Rev. 2019 Apr 11;4(4):CD007646. doi: 10.1002/14651858.CD007646.pub3. Cochrane Database Syst Rev. 2019. PMID: 30970390 Free PMC article.
-
Extended-interval Dosing of Gentamicin in Premature Neonates Born at <32 Weeks' Gestation and >7 Days of age.Clin Ther. 2017 Jun;39(6):1233-1241. doi: 10.1016/j.clinthera.2017.05.343. Epub 2017 May 31. Clin Ther. 2017. PMID: 28579209
-
Extended-interval dosing of gentamicin for treatment of neonatal sepsis in developed and developing countries.J Health Popul Nutr. 2008 Jun;26(2):163-82. J Health Popul Nutr. 2008. PMID: 18686550 Free PMC article. Review.
-
Simplified antibiotic regimens for treatment of clinical severe infection in the outpatient setting when referral is not possible for young infants in Pakistan (Simplified Antibiotic Therapy Trial [SATT]): a randomised, open-label, equivalence trial.Lancet Glob Health. 2017 Feb;5(2):e177-e185. doi: 10.1016/S2214-109X(16)30335-7. Epub 2016 Dec 15. Lancet Glob Health. 2017. PMID: 27988146 Free PMC article. Clinical Trial.
Cited by
-
Evaluation of Dosing Guidelines for Gentamicin in Neonates and Children.Antibiotics (Basel). 2023 Apr 25;12(5):810. doi: 10.3390/antibiotics12050810. Antibiotics (Basel). 2023. PMID: 37237713 Free PMC article.
-
Baseline Characteristics and Maintenance Therapy Choice on Symptom Control, Reliever Use, Exacerbation Risk in Moderate-Severe Asthma: A Clinical Modelling and Simulation Study.Adv Ther. 2024 Nov;41(11):4065-4088. doi: 10.1007/s12325-024-02962-2. Epub 2024 Sep 6. Adv Ther. 2024. PMID: 39240503 Free PMC article.
References
-
- African Neonatal Sepsis Trial Group (2013). Simplified regimens for management of neonates and young infants with severe infection when hospital admission is not possible: study protocol for a randomized, open-label equivalence trial. Pediatr. Infect. Dis. J. 32 (Suppl. 1), S26–S32. 10.1097/INF.0b013e31829ff7d1 - DOI - PMC - PubMed
-
- African Neonatal Sepsis Trial Tshefu A., Lokangaka A., Ngaima S., Engmann C., Esamai F., et al. (2015). Simplified antibiotic regimens compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with clinical signs of possible serious bacterial infection when referral is not possible: a randomised, open-label, equivalence trial. Lancet 385 (9979), 1767–1776. 10.1016/S0140-6736(14)62284-4 - DOI - PubMed
-
- Baqui A. H., Saha S. K., Ahmed A. S., Shahidullah M., Quasem I., Roth D. E., et al. (2015). Safety and efficacy of alternative antibiotic regimens compared with 7 day injectable procaine benzylpenicillin and gentamicin for outpatient treatment of neonates and young infants with clinical signs of severe infection when referral is not possible: a randomised, open-label, equivalence trial. Lancet Glob. Health 3 (5), e279–87. 10.1016/S2214-109X(14)70347-X - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

