Effect of Micelle-Incorporated Cisplatin With Sizes Ranging From 8 to 40 nm for the Therapy of Lewis Lung Carcinoma
- PMID: 33762955
- PMCID: PMC7982401
- DOI: 10.3389/fphar.2021.632877
Effect of Micelle-Incorporated Cisplatin With Sizes Ranging From 8 to 40 nm for the Therapy of Lewis Lung Carcinoma
Abstract
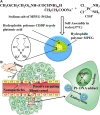
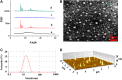
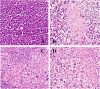
Insufficient transport of therapeutic cargo into tumor bed is a bottleneck in cancer nanomedicine. Block copolymers are promising carriers with smaller particle size by ratio modification. Here, we constructed cisplatin nanoparticles with sizes ranging from 8 to 40 nm to study the permeability and therapy of Lewis lung carcinoma. We synthesized methoxypoly(ethylene glycol)2000-block poly(L-glutamic acid sodium salt)1979 loading cisplatin through complexation reaction. The cisplatin nanomedicine has high drug loading and encapsulation efficiency. In vitro data demonstrated that cisplatin nanoparticles had equivalent growth-inhibiting effects on Lewis lung carcinoma cells compared to free cisplatin. In vivo evidences showed cisplatin nanoparticles had superior antitumor effects on the Lewis lung carcinoma mouse model with no obvious side effects. All results indicated that optimizing the ratio of block copolymers to obtain smaller sized nanomedicine could act as a promising strategy for overcoming the inadequate accumulation in poorly vascularized tumors.
Keywords: block copolymers; cisplatin; drug delivery; lung cancer; nanomedicine; nanoparticles.
Copyright © 2021 Wang, Li, Zhang, Li, Yang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
LinkOut - more resources
Full Text Sources
Other Literature Sources

