Phasic Neuronal Firing in the Rodent Nucleus of the Solitary Tract ex vivo
- PMID: 33762969
- PMCID: PMC7982836
- DOI: 10.3389/fphys.2021.638695
Phasic Neuronal Firing in the Rodent Nucleus of the Solitary Tract ex vivo
Abstract
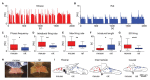
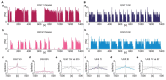
Phasic pattern of neuronal activity has been previously described in detail for magnocellular vasopressin neurons in the hypothalamic paraventricular and supraoptic nuclei. This characteristic bistable pattern consists of alternating periods of electrical silence and elevated neuronal firing, implicated in neuropeptide release. Here, with the use of multi-electrode array recordings ex vivo, we aimed to study the firing pattern of neurons in the nucleus of the solitary tract (NTS) - the brainstem hub for homeostatic, cardio-vascular, and metabolic processes. Our recordings from the mouse and rat hindbrain slices reveal the phasic activity pattern to be displayed by a subset of neurons in the dorsomedial NTS subjacent to the area postrema (AP), with the inter-spike interval distribution closely resembling that reported for phasic magnocellular vasopressin cells. Additionally, we provide interspecies comparison, showing higher phasic frequency and firing rate of phasic NTS cells in mice compared to rats. Further, we describe daily changes in their firing rate and pattern, peaking at the middle of the night. Last, we reveal these phasic cells to be sensitive to α 2 adrenergic receptors activation and to respond to electrical stimulation of the AP. This study provides a comprehensive description of the phasic neuronal activity in the rodent NTS and identifies it as a potential downstream target of the AP noradrenergic system.
Keywords: brainstem; multi-electrode array; nucleus of the solitary tract; phasic; timekeeping.
Copyright © 2021 Chrobok, Wojcik, Klich, Pradel, Lewandowski and Piggins.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Armstrong D. M., Pickel V. M., Joh T. H., Reis D. J., Miller R. J. (1981). Immunocytochemical localization of catecholamine synthesizing enzymes and neuropeptides in area postrema and medial nucleus tractus solitarius of rat brain. J. Comp. Neurol. 196, 505–517. 10.1002/cne.901960312, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

