Descriptive Analysis of Real-World Data on Fingolimod Long-Term Treatment of Young Adult RRMS Patients
- PMID: 33763018
- PMCID: PMC7982917
- DOI: 10.3389/fneur.2021.637107
Descriptive Analysis of Real-World Data on Fingolimod Long-Term Treatment of Young Adult RRMS Patients
Abstract
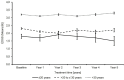
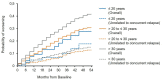
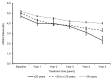
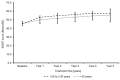
Background: Fingolimod (Gilenya®) is approved for adult and pediatric patients with highly active relapsing-remitting multiple sclerosis (RRMS). Objectives: The objective was to describe the effectiveness of fingolimod in young adults compared to older patients in clinical practice. Methods: PANGAEA is the largest prospective, multi-center, non-interventional, long-term study evaluating fingolimod in RRMS. We descriptively analyzed demographics, MS characteristics, and severity in two subgroups of young adults (≤20 and >20 to ≤30 years) and older patients (>30 years). Results: Young adults had lower Expanded Disability Status Scale (EDSS) scores compared to older patients (1.8 and 2.3 vs. 3.2) at baseline. The mean EDSS scores remained stable over 5 years in all subgroups. Young adults had higher annual relapse rates (2.0 and 1.7 vs. 1.4) at study entry, which were reduced by approximately 80% in all subgroups over 5 years. The proportion of patients with no clinical disease activity in year 4 was 52.6 and 73.4 vs. 66.9% in patients ≤20, >20 to ≤30 years and >30 years, respectively. The symbol digit modalities test score increased by 15.25 ± 8.3 and 8.3 ± 11.3 (mean ± SD) from baseline in patients >20 to ≤30 and >30 years. Conclusions: Real-world evidence suggests a long-term treatment benefit of fingolimod in young RRMS patients.
Keywords: RRMS; early treatment; fingolimod; long-term study; real-world evidence; young adults.
Copyright © 2021 Ziemssen, Albrecht, Haas, Klotz, Lang, Lassek, Schmidt, Ettle and Schulze-Topphoff.
Conflict of interest statement
TZ has received personal compensation for participating on advisory boards, trial steering committees, and data and safety monitoring committees as well as for scientific talks and project support from Bayer HealthCare, Biogen, Celgene, Genzyme, Merck, Novartis, Roche, Sanofi, and Teva. HA has received travel grants, speaker's honoraria, and consultancy fees from Teva, Merck Serono, Genzyme, Sanofi, Novartis, Bayer, and Biogen. JH has received honorarium from Biogen Idec, Merck Serono, Bayer Schering, Teva-Aventis, Novartis, and Octapharma. LK received compensation for serving on scientific advisory boards for Genzyme and Novartis. She received speaker honoraria and travel support from Novartis, Merck Serono, and CSL Behring and receives research support from Novartis and Biogen. ML has received research support from Novartis. CL has received travel grants, speaker's honoraria, financial research support, and consultancy fees from Teva, Merck Serono, Genzyme, Sanofi, Novartis, Bayer, and Biogen. SS has received speaking honoraria and travel compensations and has served on advisory boards for BayerVital, Biogen, MerckSerono, Novartis, and Teva. BE and US-T are employees of Novartis Pharma GmbH, Nuremberg, Germany. The authors declare that this non-interventional study was sponsored and funded by Novartis Pharma GmbH. The role of the sponsor and funder included protocol development, study administration, data management, data analysis, and manuscript preparation. Novartis Pharma GmbH further funded the medical writing support.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

