Phage-Mediated Control of Flavobacterium psychrophilum in Aquaculture: In vivo Experiments to Compare Delivery Methods
- PMID: 33763046
- PMCID: PMC7983945
- DOI: 10.3389/fmicb.2021.628309
Phage-Mediated Control of Flavobacterium psychrophilum in Aquaculture: In vivo Experiments to Compare Delivery Methods
Abstract
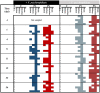
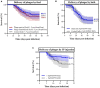
Phage-based approaches have gained increasing interest as sustainable alternative strategies to antibiotic treatment or as prophylactic measures against disease outbreaks in aquaculture. The potential of three methods (oral, bath, and injection) for delivering a two-component phage mixture to rainbow trout fry for controlling Flavobacterium psychrophilum infections and reduce fish mortality was investigated using bacteriophages FpV4 and FPSV-D22. For the oral administration experiment, bacteriophages were applied on feed pellets by spraying (1.6 × 108 PFU g-1) or by irreversible immobilization (8.3 × 107 PFU g-1), using the corona discharge technology (Fixed Phage Ltd.). The fish showed normal growth for every group and no mortality was observed prior to infection as well as in control groups during the infection. Constant detection of phages in the intestine (∼103 PFU mg-1) and more sporadic occurrence in kidney, spleen, and brain was observed. When fish were exposed to F. psychrophilum, no significant effect on fish survival, nor a direct impact on the number of phages in the sampled organs, were detected. Similarly, no significant increase in fish survival was detected when phages were delivered by bath (1st and 2nd bath: ∼106 PFU ml-1; 3rd bath: ∼105 PFU ml-1). However, when phages FpV4 and FPSV-D22 (1.7 × 108 PFU fish-1) were administered by intraperitoneal injection 3 days after the bacterial challenge, the final percent survival observed in the group injected with bacteriophages FpV4 and FPSV-D22 (80.0%) was significantly higher than in the control group (56.7%). The work demonstrates the delivery of phages to fish organs by oral administration, but also suggests that higher phage dosages than the tested ones may be needed on feed pellets to offer fish an adequate protection against F. psychrophilum infections.
Keywords: Flavobacterium psychrophilum; bacteriophages; phage-therapy; rainbow trout fry (Oncorhynchus mykiss); rainbow trout fry syndrome (RTFS).
Copyright © 2021 Donati, Dalsgaard, Sundell, Castillo, Er-Rafik, Clark, Wiklund, Middelboe and Madsen.
Conflict of interest statement
JC was employed by the company Fixed Phage Ltd. (Glasgow, United Kingdom). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Amend D. F. (1981). Potency testing of fish vaccines. Develop. Biol. Standard 49 447–454.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

