Cross Kingdom Immunity: The Role of Immune Receptors and Downstream Signaling in Animal and Plant Cell Death
- PMID: 33763054
- PMCID: PMC7982415
- DOI: 10.3389/fimmu.2020.612452
Cross Kingdom Immunity: The Role of Immune Receptors and Downstream Signaling in Animal and Plant Cell Death
Abstract
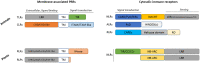
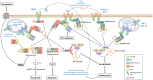
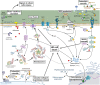
Both plants and animals are endowed with sophisticated innate immune systems to combat microbial attack. In these multicellular eukaryotes, innate immunity implies the presence of cell surface receptors and intracellular receptors able to detect danger signal referred as damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). Membrane-associated pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), C-type lectin receptors (CLRs), receptor-like kinases (RLKs), and receptor-like proteins (RLPs) are employed by these organisms for sensing different invasion patterns before triggering antimicrobial defenses that can be associated with a form of regulated cell death. Intracellularly, animals nucleotide-binding and oligomerization domain (NOD)-like receptors or plants nucleotide-binding domain (NBD)-containing leucine rich repeats (NLRs) immune receptors likely detect effectors injected into the host cell by the pathogen to hijack the immune signaling cascade. Interestingly, during the co-evolution between the hosts and their invaders, key cross-kingdom cell death-signaling macromolecular NLR-complexes have been selected, such as the inflammasome in mammals and the recently discovered resistosome in plants. In both cases, a regulated cell death located at the site of infection constitutes a very effective mean for blocking the pathogen spread and protecting the whole organism from invasion. This review aims to describe the immune mechanisms in animals and plants, mainly focusing on cell death signaling pathways, in order to highlight recent advances that could be used on one side or the other to identify the missing signaling elements between the perception of the invasion pattern by immune receptors, the induction of defenses or the transmission of danger signals to other cells. Although knowledge of plant immunity is less advanced, these organisms have certain advantages allowing easier identification of signaling events, regulators and executors of cell death, which could then be exploited directly for crop protection purposes or by analogy for medical research.
Keywords: NOD-like receptors; Toll-like receptors; damage-associated molecular patterns; hypersensitive response; pathogen-associated molecular patterns; pattern recognition receptors; regulated cell death.
Copyright © 2021 Roudaire, Héloir, Wendehenne, Zadoroznyj, Dubrez and Poinssot.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

