Exploiting Genic Male Sterility in Rice: From Molecular Dissection to Breeding Applications
- PMID: 33763090
- PMCID: PMC7982899
- DOI: 10.3389/fpls.2021.629314
Exploiting Genic Male Sterility in Rice: From Molecular Dissection to Breeding Applications
Abstract
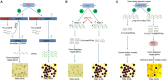
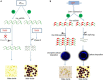
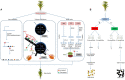
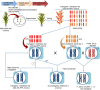
Rice (Oryza sativa L.) occupies a very salient and indispensable status among cereal crops, as its vast production is used to feed nearly half of the world's population. Male sterile plants are the fundamental breeding materials needed for specific propagation in order to meet the elevated current food demands. The development of the rice varieties with desired traits has become the ultimate need of the time. Genic male sterility is a predominant system that is vastly deployed and exploited for crop improvement. Hence, the identification of new genetic elements and the cognizance of the underlying regulatory networks affecting male sterility in rice are crucial to harness heterosis and ensure global food security. Over the years, a variety of genomics studies have uncovered numerous mechanisms regulating male sterility in rice, which provided a deeper and wider understanding on the complex molecular basis of anther and pollen development. The recent advances in genomics and the emergence of multiple biotechnological methods have revolutionized the field of rice breeding. In this review, we have briefly documented the recent evolution, exploration, and exploitation of genic male sterility to the improvement of rice crop production. Furthermore, this review describes future perspectives with focus on state-of-the-art developments in the engineering of male sterility to overcome issues associated with male sterility-mediated rice breeding to address the current challenges. Finally, we provide our perspectives on diversified studies regarding the identification and characterization of genic male sterility genes, the development of new biotechnology-based male sterility systems, and their integrated applications for hybrid rice breeding.
Keywords: anther and pollen development; biotechnology based male sterility systems; genic male sterility; hybrid breeding; regulatory mechanism; rice.
Copyright © 2021 Abbas, Yu, Sun, Yang, Chen, Cheng and Cao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

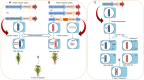
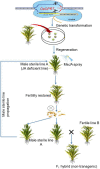
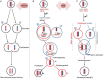
References
-
- Akasaka M., Taniguchi Y., Oshima M., Abe K., Tabei Y., Tanaka J. (2018). Development of transgenic male-sterile rice by using anther-specific promoters identified by comprehensive screening of the gene expression profile database ‘RiceXPro’. Breed. Sci. 68 420–431. 10.1270/jsbbs.18019 - DOI - PMC - PubMed
-
- Anderson S. N., Johnson C. S., Jones D. S., Conrad L. J., Gou X., Russell S. D., et al. (2013). Transcriptomes of isolated Oryza sativa gametes characterized by deep sequencing: evidence for distinct sex-dependent chromatin and epigenetic states before fertilization. Plant J. 76 729–741. 10.1111/tpj.12336 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

