Graphene Oxide/Polyvinyl Alcohol/Fe3O4 Nanocomposite: An Efficient Adsorbent for Co(II) Ion Removal
- PMID: 33763287
- PMCID: PMC7964109
- DOI: 10.1155/2021/6670913
Graphene Oxide/Polyvinyl Alcohol/Fe3O4 Nanocomposite: An Efficient Adsorbent for Co(II) Ion Removal
Abstract
In this work, an effective nanocomposite-based adsorbent directed to adsorb cobalt (Co2+) ion was successfully synthesized from graphene oxide (GO), polyvinyl alcohol (PVA), and magnetite (Fe3O4) nanoparticles via a coprecipitation technique. The synthesized GO/PVA/Fe3O4 nanocomposite was applied for Co2+ ion removal with the optimized working conditions including 100 min of contact time, 0.01 g of adsorbent dosage, pH of 5.2, and 50°C of temperature. The investigation of adsorption kinetics showed that the adsorption of Co2+ ion onto the GO/PVA/Fe3O4 nanocomposite followed the pseudo-second-order kinetic model with the rate constant k2 being 0.0026 (g mg-1·min-1). The Langmuir model is suitable to describe the adsorption of Co2+ ion onto the GO/PVA/Fe3O4 nanocomposite with the maximum sorption capacity (q max) reaching 373.37 mg·g-1. The obtained results also indicated that the GO/PVA/Fe3O4 nanocomposite can adsorb/regenerate for at least 5 cycles with a little reduction in removal efficiency. Therefore, we believe that the GO/PVA/Fe3O4 nanocomposite could be used as a potential adsorbent for heavy metal treatment in terms of high adsorption capacity, fast adsorption rate, and recyclability.
Copyright © 2021 Thu Dieu Le et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
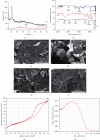


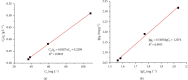

References
-
- Awwad A., Amer M., Al-aqarbeh M. TIO2-Kaolinite nanocomposite prepared from the Jordanian kaolin clay: adsorption and thermodynamics of Pb(II) and Cd(II) ions in aqueous solution. Chemistry International. 2020;6:168–178.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

