Daratumumab as Single Agent in Relapsed/Refractory Myeloma Patients: A Retrospective Real-Life Survey
- PMID: 33763359
- PMCID: PMC7982826
- DOI: 10.3389/fonc.2021.624405
Daratumumab as Single Agent in Relapsed/Refractory Myeloma Patients: A Retrospective Real-Life Survey
Abstract
Background: The anti-CD38 monoclonal antibody daratumumab is approved as a single agent for the treatment of patients with relapsed/refractory multiple myeloma (RRMM) who received at least three prior lines of therapy, including proteasome inhibitor and immunomodulatory agent. A retrospective multicentric study was designed to evaluate feasibility, tolerability, and efficacy of daratumumab in monotherapy in RRMM.
Methods: This study included 44 consecutive RRMM patients that underwent daratumumab monotherapy after a median number of four prior therapies (range 2-9). Patients were treated in seven Sicilian centers, as part of Sicilian Myeloma Network and three Calabrian centers outside of controlled clinical trials from August 2016 through July 2020.
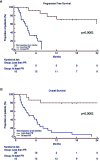
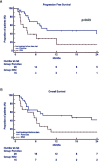
Results: The regimen was well tolerated with few grade 3-4 haematological and rare non-haematological adverse events, such as pneumonia. Definitive discontinuation was due to disease progression in 25 (57%) patients. Since three patients did not complete at least one full cycle, a total of 41 patients was evaluated for response. Overall response rate was 37%, and the disease control rate (stable disease or better) was high (73%). The best achieved responses within 6 months were very good partial remission or better (27%), partial remission (10%), minimal response (14%) and stable disease (22%). After a median follow up of 7.8 months, median progression free survival (PFS) was 7.2 months and overall survival (OS) 7.8 months. Univariate analysis showed that patients with PR or better after 6 months of therapy had longer median PFS and OS (respectively 29.5 vs 3.6 months, p=0.0001 and 30.6 vs 3.9 months p=0.0001), confirmed by multivariate analysis. Furthermore, standard cytogenetic risk and biochemical relapse type had prolonged median PFS, but not OS (respectively unreached vs 2.6, p=0.03 and 23.9 vs 6.2, p=0.05) in both univariate and multivariate analysis. Additionally, univariate analysis showed that patients treated with carfilzomib-lenalidomide-dexamethasone prior to daratumumab had significantly shorter PFS compared to pomalidomide-dexamethasone (3.4 months vs 9.3 months, p=0.03), that multivariate analysis failed to confirm.
Conclusions: Our findings indicate that daratumumab as single agent is safe and well-tolerated regimen in real-life, associated to prolonged PFS and OS in responding patients. No new safety signals were identified.
Keywords: daratumumab; immunotherapy; multiple myeloma; relapsed/refractory; salvage treatment.
Copyright © 2021 Markovic, Romano, Del Fabro, Bellofiore, Bulla, Parisi, Leotta, Gentile, Cangialosi, Vincelli, Mineo, Rossi, Poidomani, Uccello, Maugeri, Mannina, Innao, Di Raimondo and Conticello.
Conflict of interest statement
CC, FR, AR, and UM received honoraria from Amgen. CC, FR, and AR received honoraria from Celgene. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia (2012) 26(1):149–57. 10.1038/leu.2011.196 - DOI - PMC - PubMed
-
- Miguel JS, Weisel K, Moreau P, Lacy M, Song K, Delforge M, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol (2013) 14(11):1055–66. 10.1016/S1470-2045(13)70380-2 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

