A Parallel Perifusion Slide From Glass for the Functional and Morphological Analysis of Pancreatic Islets
- PMID: 33763408
- PMCID: PMC7982818
- DOI: 10.3389/fbioe.2021.615639
A Parallel Perifusion Slide From Glass for the Functional and Morphological Analysis of Pancreatic Islets
Abstract
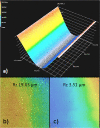
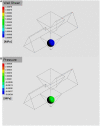
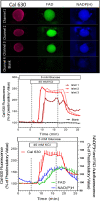
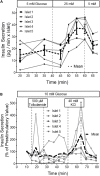
An islet-on-chip system in the form of a completely transparent microscope slide optically accessible from both sides was developed. It is made from laser-structured borosilicate glass and enables the parallel perifusion of five microchannels, each containing one islet precisely immobilized in a pyramidal well. The islets can be in inserted via separate loading windows above each pyramidal well. This design enables a gentle, fast and targeted insertion of the islets and a reliable retention in the well while at the same time permitting a sufficiently fast exchange of the media. In addition to the measurement of the hormone content in the fractionated efflux, parallel live cell imaging of the islet is possible. By programmable movement of the microscopic stage imaging of five wells can be performed. The current chip design ensures sufficient time resolution to characterize typical parameters of stimulus-secretion coupling. This was demonstrated by measuring the reaction of the islets to stimulation by glucose and potassium depolarization. After the perifusion experiment islets can be removed for further analysis. The live-dead assay of the removed islets confirmed that the process of insertion and removal was not detrimental to islet structure and viability. In conclusion, the present islet-on-chip design permits the practical implementation of parallel perifusion experiments on a single and easy to load glass slide. For each immobilized islet the correlation between secretion, signal transduction and morphology is possible. The slide concept allows the scale-up to even higher degrees of parallelization.
Keywords: NAD(P)H- and FAD-autofluorescence; borosilicate glass; calcium; femtosecond laser-structuring; insulin secretion; islet of langerhans; microfluidic perifusion system.
Copyright © 2021 Schulze, Mattern, Erfle, Brüning, Scherneck, Dietzel and Rustenbeck.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
-
- Ahlqvist E., Storm P., Käräjämäki A., Martinell M., Dorkhan M., Carlsson A., et al. (2018). Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 6 361–369. 10.1016/s2213-8587(18)30051-2 - DOI - PubMed
-
- Ashcroft F. M., Rorsman P. (1989). Electrophysiology of the pancreatic beta-cell. Prog. Biophys. Mol. Biol. 54 87–143. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

