Synthesis, Molecular Docking Analysis, and Evaluation of Antibacterial and Antioxidant Properties of Stilbenes and Pinacol of Quinolines
- PMID: 33763647
- PMCID: PMC7946464
- DOI: 10.1155/2021/6635270
Synthesis, Molecular Docking Analysis, and Evaluation of Antibacterial and Antioxidant Properties of Stilbenes and Pinacol of Quinolines
Abstract
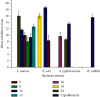
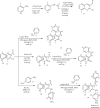
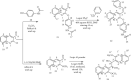
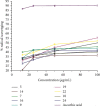
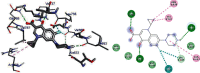
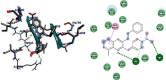
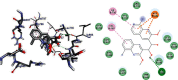
Emergence of antimicrobial resistance to standard commercial drugs has become a critical public health concern worldwide. Hence, novel antimicrobials with improved biological activities are urgently needed. In this regard, a series of quinoline-stilbene derivatives were synthesized from substituted quinoline and benzyltriphenylphosphonium chloride using Wittig reaction. Furthermore, a novel pinacol of quinoline was synthesized by pinacolinazation of 2-methoxyquinoline-3-carbaldehyde which was achieved by aluminum powder-potassium hydroxide reagent combination at ambient temperature in methanol. The structures of the synthesized compounds were established based on their spectral data. The antibacterial activities of the synthesized compounds were evaluated in vitro by the paper disc diffusion method against two Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis) and two Gram-negative bacteria (Escherichia coli and Salmonella typhimurium). The best activity was displayed by compound 19 against E. coli with an inhibition zone of 16.0 ± 0.82 mm and 14.67 ± 0.94 mm at 500 and 250 μg/mL, respectively. This is close to ciprofloxacin which is used as a positive control. The results of in silico molecular docking evaluation of the compounds against E. coli DNA gyraseB were in good agreement with the in vitro antibacterial analysis. Compounds 19 (-6.9 kcal/mol) and 24 (-7.1 kcal/mol) showed the maximum binding affinity close to ciprofloxacin (-7.3 kcal/mol) used as positive control. Therefore, the antibacterial activity displayed by these compounds is encouraging for further investigation to improve the activities of quinoline-stilbenes by incorporating various bioisosteric groups in one or more positions of the phenyl nuclei for their potential pharmacological use. Findings of the DPPH radical scavenging assay indicated that some of the quinolone stilbenes and pinacol possess moderate antioxidant properties compared to ascorbic acid used as a natural antioxidant.
Copyright © 2021 Zeleke Digafie et al.
Conflict of interest statement
The authors disclose that there are no conflicts of interest regarding the publication of this paper.
Figures


References
-
- Desai N. C., Patel B. Y., Dave B. P. Synthesis and antimicrobial activity of novel quinoline derivatives bearing pyrazoline and pyridine analogues. Medicinal Chemistry Research. 2017;26(1):109–119. doi: 10.1007/s00044-016-1732-6. - DOI
-
- Verbanac D., Malik R., Chand M., et al. Synthesis and evaluation of antibacterial and antioxidant activity of novel 2-phenyl-quinoline analogs derivatized at position 4 with aromatically substituted 4H-1,2,4-triazoles. Journal of Enzyme Inhibition and Medicinal Chemistry. 2016;31(2):104–110. doi: 10.1080/14756366.2016.1190714. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
