Protein/AS01B vaccination elicits stronger, more Th2-skewed antigen-specific human T follicular helper cell responses than heterologous viral vectors
- PMID: 33763653
- PMCID: PMC7974546
- DOI: 10.1016/j.xcrm.2021.100207
Protein/AS01B vaccination elicits stronger, more Th2-skewed antigen-specific human T follicular helper cell responses than heterologous viral vectors
Abstract
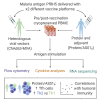
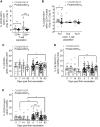
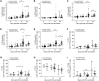
Interactions between B cells and CD4+ T follicular helper (Tfh) cells are key determinants of humoral responses. Using samples from clinical trials performed with the malaria vaccine candidate antigen Plasmodium falciparum merozoite protein (PfRH5), we compare the frequency, phenotype, and gene expression profiles of PfRH5-specific circulating Tfh (cTfh) cells elicited by two leading human vaccine delivery platforms: heterologous viral vector prime boost and protein with AS01B adjuvant. We demonstrate that the protein/AS01B platform induces a higher-magnitude antigen-specific cTfh cell response and that this correlates with peak anti-PfRH5 IgG concentrations, frequency of PfRH5-specific memory B cells, and antibody functionality. Furthermore, our data indicate a greater Th2/Tfh2 skew within the polyfunctional response elicited following vaccination with protein/AS01B as compared to a Th1/Tfh1 skew with viral vectors. These data highlight the impact of vaccine platform on the cTfh cell response driving humoral immunity, associating a high-magnitude, Th2-biased cTfh response with potent antibody production.
Trial registration: ClinicalTrials.gov NCT02927145.
Keywords: AS01; T follicular helper cells; Tfh cells; Th1; Th2; adaptive immunity; antibody; clinical trials; heterologous viral vectors; malaria; vaccines.
© 2021 The Authors.
Conflict of interest statement
S.J.D. is a named inventor on patent applications relating to PfRH5 and/or other malaria vaccines and immunization regimens. A.M.M. has an immediate family member who is listed as an inventor on patents relating to PfRH5 and/or other malaria vaccines and immunization regimens.
Figures



References
-
- Jin J., Tarrant R.D., Bolam E.J., Angell-Manning P., Soegaard M., Pattinson D.J., Dulal P., Silk S.E., Marshall J.M., Dabbs R.A. Production, quality control, stability, and potency of cGMP-produced Plasmodium falciparum RH5.1 protein vaccine expressed in Drosophila S2 cells. NPJ Vaccines. 2018;3:32. - PMC - PubMed
-
- O’Donnell K., Marzi A. The Ebola virus glycoprotein and its immune responses across multiple vaccine platforms. Expert Rev. Vaccines. 2020;19:267–277. - PubMed
-
- Heineman T.C., Cunningham A., Levin M. Understanding the immunology of Shingrix, a recombinant glycoprotein E adjuvanted herpes zoster vaccine. Curr. Opin. Immunol. 2019;59:42–48. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

