Plasma gelsolin modulates the production and fate of IL-1β-containing microparticles following high-pressure exposure and decompression
- PMID: 33764168
- PMCID: PMC8354825
- DOI: 10.1152/japplphysiol.01062.2020
Plasma gelsolin modulates the production and fate of IL-1β-containing microparticles following high-pressure exposure and decompression
Abstract
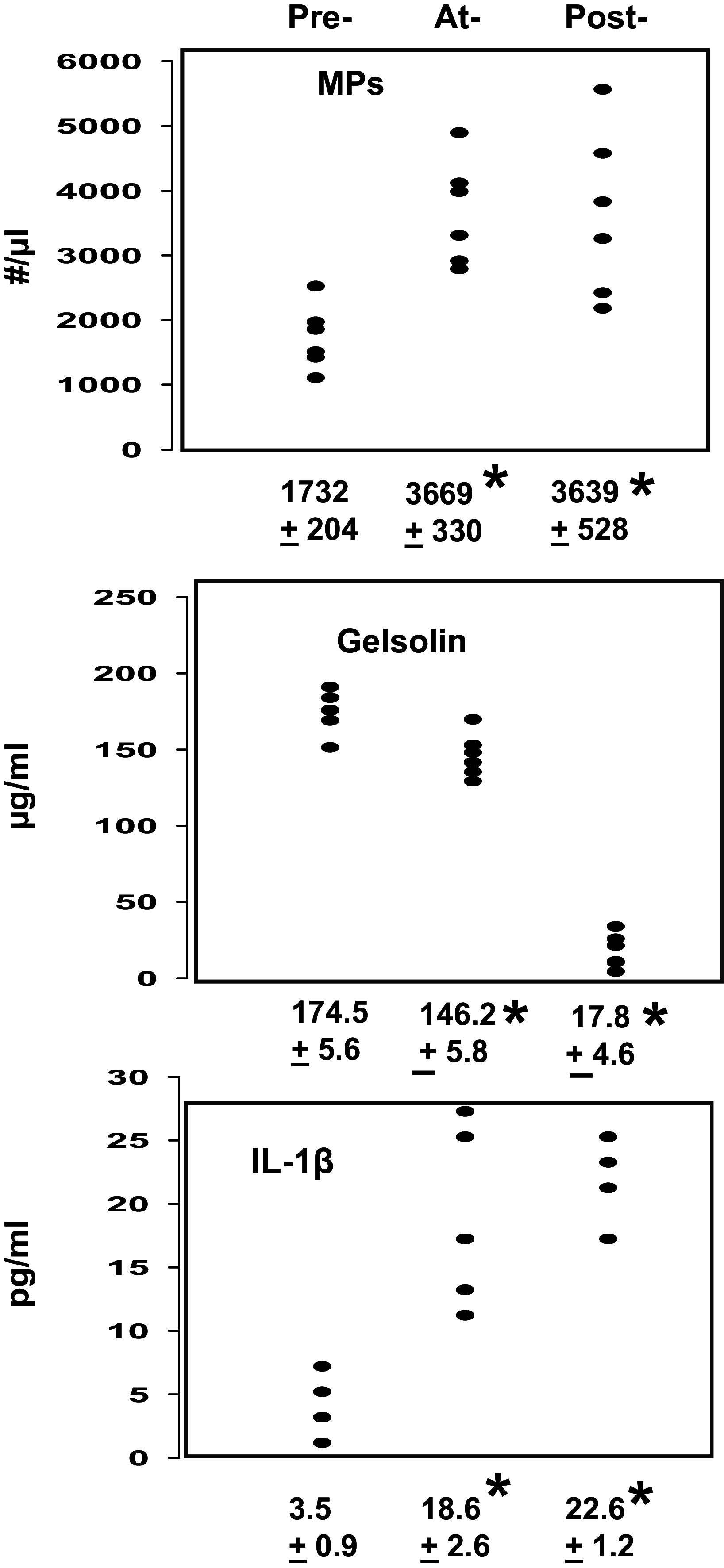
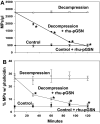
Plasma gelsolin (pGSN) levels fall in association with diverse inflammatory conditions. We hypothesized that pGSN would decrease due to the stresses imposed by high pressure and subsequent decompression, and repletion would ameliorate injuries in a murine decompression sickness (DCS) model. Research subjects were found to exhibit a modest decrease in pGSN level while at high pressure and a profound decrease after decompression. Changes occurred concurrent with elevations of circulating microparticles (MPs) carrying interleukin (IL)-1β. Mice exhibited a comparable decrease in pGSN after decompression along with elevations of MPs carrying IL-1β. Infusion of recombinant human (rhu)-pGSN into mice before or after pressure exposure abrogated these changes and prevented capillary leak in brain and skeletal muscle. Human and murine MPs generated under high pressure exhibited surface filamentous actin (F-actin) to which pGSN binds, leading to particle lysis. In addition, human neutrophils exposed to high air pressure exhibit an increase in surface F-actin that is diminished by rhu-pGSN resulting in inhibition of MP production. Administration of rhu-pGSN may have benefit as prophylaxis or treatment for DCS.NEW & NOTEWORTHY Inflammatory microparticles released in response to high pressure and decompression express surface filamentous actin. Infusion of recombinant human plasma gelsolin lyses these particles in decompressed mice and ameliorates particle-associated vascular damage. Human neutrophils also respond to high pressure with an increase in surface filamentous actin and microparticle production, and these events are inhibited by plasma gelsolin. Gelsolin infusion may have benefit as prophylaxis or treatment for decompression sickness.
Keywords: decompression sickness; interleukin-1β; microparticles; neutrophils; oxidative stress.
Conflict of interest statement
S.L. Levinson and M.J. DiNubile are employed by and own stock in BioAegis Therapeutics, which is developing recombinant human plasma gelsolin for clinical use. Other authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Recombinant human plasma gelsolin suppresses persistent neuroinflammation and restores hippocampal neurogenesis in murine model of decompression sickness.J Neurophysiol. 2024 Dec 1;132(6):1877-1886. doi: 10.1152/jn.00332.2024. Epub 2024 Oct 30. J Neurophysiol. 2024. PMID: 39475490
-
Microparticle-induced vascular injury in mice following decompression is inhibited by hyperbaric oxygen: effects on microparticles and interleukin-1β.J Appl Physiol (1985). 2019 Apr 1;126(4):1006-1014. doi: 10.1152/japplphysiol.01109.2018. Epub 2019 Feb 14. J Appl Physiol (1985). 2019. PMID: 30763157
-
Provocative decompression causes diffuse vascular injury in mice mediated by microparticles containing interleukin-1β.J Appl Physiol (1985). 2018 Oct 1;125(4):1339-1348. doi: 10.1152/japplphysiol.00620.2018. Epub 2018 Aug 16. J Appl Physiol (1985). 2018. PMID: 30113270
-
Exosomal Plasma Gelsolin Is an Immunosuppressive Mediator in the Ovarian Tumor Microenvironment and a Determinant of Chemoresistance.Cells. 2022 Oct 20;11(20):3305. doi: 10.3390/cells11203305. Cells. 2022. PMID: 36291171 Free PMC article. Review.
-
Emerging roles of plasma gelsolin in tumorigenesis and modulating the tumor microenvironment.Kaohsiung J Med Sci. 2022 Sep;38(9):819-825. doi: 10.1002/kjm2.12578. Epub 2022 Aug 9. Kaohsiung J Med Sci. 2022. PMID: 35942641 Free PMC article. Review.
Cited by
-
Inert Gas Mild Pressure Action on Healthy Humans: The "IPA" Study.Int J Mol Sci. 2024 Nov 10;25(22):12067. doi: 10.3390/ijms252212067. Int J Mol Sci. 2024. PMID: 39596136 Free PMC article.
-
Oxygen Variations-Insights into Hypoxia, Hyperoxia and Hyperbaric Hyperoxia-Is the Dose the Clue?Int J Mol Sci. 2023 Aug 30;24(17):13472. doi: 10.3390/ijms241713472. Int J Mol Sci. 2023. PMID: 37686277 Free PMC article.
-
Varying Oxygen Partial Pressure Elicits Blood-Borne Microparticles Expressing Different Cell-Specific Proteins-Toward a Targeted Use of Oxygen?Int J Mol Sci. 2022 Jul 17;23(14):7888. doi: 10.3390/ijms23147888. Int J Mol Sci. 2022. PMID: 35887238 Free PMC article. Clinical Trial.
-
Recombinant human plasma gelsolin suppresses persistent neuroinflammation and restores hippocampal neurogenesis in murine model of decompression sickness.J Neurophysiol. 2024 Dec 1;132(6):1877-1886. doi: 10.1152/jn.00332.2024. Epub 2024 Oct 30. J Neurophysiol. 2024. PMID: 39475490
-
Elevations of Extracellular Vesicles and Inflammatory Biomarkers in Closed Circuit SCUBA Divers.Int J Mol Sci. 2023 Mar 22;24(6):5969. doi: 10.3390/ijms24065969. Int J Mol Sci. 2023. PMID: 36983042 Free PMC article.
References
-
- Bucki R, Kulakowska A, Byfield FJ, Zendzian-Piotrowska M, Baranowski M, Marzec M, Winer JP, Ciccarelli NJ, Gorski J, Drozdowski W, Bittman R, Janmey PA. Plasma gelsolin modulates cellular response to sphingosine 1-phosphate. Am J Physiol Cell Physiol 299: C1516–C1523, 2010. doi:10.1152/ajpcell.00051.2010. - DOI - PMC - PubMed
-
- Ordija CM, Chiou TT, Yang Z, Deloid GM, de Oliveira Valdo M, Wang Z, Bedugnis A, Noah TL, Jones S, Koziel H, Kobzik L. Free actin impairs macrophage bacterial defenses via scavenger receptor MARCO interaction with reversal by plasma gelsolin. Am J Physiol Lung Cell Mol Physiol 312: L1018–L1028, 2017. doi:10.1152/ajplung.00067.2017. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

