Androgen-induced epigenetic modulations in the ovary
- PMID: 33764313
- PMCID: PMC8080881
- DOI: 10.1530/JOE-20-0578
Androgen-induced epigenetic modulations in the ovary
Abstract
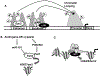
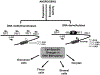
In recent years, androgens have emerged as critical regulators of female reproduction and women's health in general. While high levels of androgens in women are associated with polycystic ovary syndrome (PCOS), recent evidence suggests that a certain amount of direct androgen action through androgen receptor is also essential for normal ovarian function. Moreover, prenatal androgen exposure has been reported to cause developmental reprogramming of the fetus that manifests into adult pathologies, supporting the Developmental Origins of Health and Disease (DOHaD) hypothesis. Therefore, it has become imperative to understand the underlying mechanism of androgen actions and its downstream effects under normal and pathophysiological conditions. Over the years, there has been a lot of studies on androgen receptor function as a transcriptional regulator in the nucleus as well as androgen-induced rapid extra-nuclear signaling. Conversely, new evidence suggests that androgen actions may also be mediated through epigenetic modulation involving both the nuclear and extra-nuclear androgen signaling. This review focuses on androgen-induced epigenetic modifications in female reproduction, specifically in the ovary, and discusses emerging concepts, latest perceptions, and highlight the areas that need further investigation.
Keywords: DNA methylation; androgens; developmental programming; epigenetics; female reproduction; histone modification.
Conflict of interest statement
Figures
References
-
- ABBOTT DH, BARNETT DK, BRUNS CM & DUMESIC DA 2005. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update, 11, 357–74. - PubMed
-
- ABBOTT DH, DUMESIC DA & FRANKS S 2002. Developmental origin of polycystic ovary syndrome - a hypothesis. J Endocrinol, 174, 1–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

