An angiogenesis-related long noncoding RNA signature correlates with prognosis in patients with hepatocellular carcinoma
- PMID: 33764367
- PMCID: PMC8026853
- DOI: 10.1042/BSR20204442
An angiogenesis-related long noncoding RNA signature correlates with prognosis in patients with hepatocellular carcinoma
Abstract
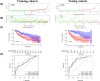
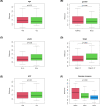
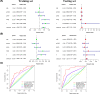
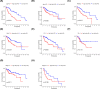
Hepatocellular carcinoma (HCC) is one of the most prevalent and lethal cancers worldwide. Neovascularization is closely related to the malignancy of tumors. We constructed a signature of angiogenesis-related long noncoding RNA (lncRNA) to predict the prognosis of patients with HCC. The lncRNA expression matrix of 424 HCC patients was downloaded from The Cancer Genome Atlas (TCGA). First, gene set enrichment analysis (GSEA) was used to distinguish the differentially expressed genes of the angiogenesis genes in liver cancer and adjacent tissues. Next, a signature of angiogenesis-related lncRNAs was constructed using univariate and multivariate analyses, and receiver operating characteristic (ROC) curves were used to assess the accuracy. The signature and relevant clinical information were used to construct the nomogram. A 5-lncRNA signature was highly correlated with overall survival (OS) in HCC patients and performed well in evaluations using the C-index, areas under the curve, and calibration curves. In summary, the 5-lncRNA model can serve as an accurate signature to predict the prognosis of patients with liver cancer, but its mechanism of action must be further elucidated by experiments.
Keywords: GSEA; angiogenesis; mRNA signature.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

